Cationic lipid compound, composition containing same and application
A technology of cationic lipids and compounds, applied in the field of medicine, can solve the problems of increasing production, increasing toxicity, and complicating
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0186] Example 1: Synthesis of Cationic Lipid Compounds
[0187] 1. Synthesis of 2-octyldecyl 6-((4-(undecyloxy)-4-oxobutyl)(2-hydroxyethyl)amino)hexanoate (YK-001)
[0188] The synthetic route is as follows:
[0189]
[0190] Step 1: Synthesis of n-undecyl 4-bromobutyrate (YK-001-PM1)
[0191] N-undecyl alcohol (5.00g, 29.02mmol) and 4-bromobutanoic acid (5.14g, 30.78mmol) were dissolved in dichloromethane (40mL), and 1-(3-dimethylaminopropyl) was added to the above solution -3-Ethylcarbodiimide hydrochloride (6.67g, 34.82mmol) and 4-dimethylaminopyridine (177mg, 1.45mmol), stirred and reacted at 30~35°C for 8 hours. After the reaction was completed, the reaction solution was washed with saturated sodium carbonate, saturated brine, and washed with Na 2 SO 4 dry. The mixture was filtered, and the filtrate was concentrated under reduced pressure in vacuo. The residue was purified by silica gel chromatography to obtain n-undecyl 4-bromobutyrate (6.68 g, 20.79 mmol, 71.6...
Embodiment 2
[0324] Example 2: Optimization of Preparation Conditions for Lipid Nanoparticles (LNP Preparations)
[0325] 1. Optimizing the ratio of carrier (liposome) to mRNA
[0326]
[0327] The cationic lipid compound YK-009 synthesized in Example 1 was mixed with DSPC (Aiweituo (Shanghai) Pharmaceutical Technology Co., Ltd.), cholesterol (Aiweituo (Shanghai) Pharmaceutical Technology Co., Ltd.) and DMG-PEG2000 according to 50: Dissolve in ethanol at a molar ratio of 10:38.5:1.5 to prepare an ethanolic lipid solution. Quickly add ethanol lipid solution to citrate buffer (pH=4~5) by ethanol injection method, and vortex for 30s for later use. Dilute eGFP-mRNA in citrate buffer (pH=4~5) to obtain mRNA aqueous solution. A certain volume of liposome solution and mRNA aqueous solution were used to prepare liposomes at a weight ratio of total lipid to mRNA of 4: 1, 10: 1, 16: 1, 24: 1, and 30: 1, respectively. Ultrasound at 25°C for 15 minutes (ultrasonic frequency 40kHz, ultrasonic pow...
Embodiment 3
[0338] Embodiment 3: LNP preparation cell transfection experiment of eGFP-mRNA
[0339] Cell recovery and passage: 293T cells were recovered and cultured in a culture dish to reach the required number of cells.
[0340] Seed plate: Digest and count the cells in the culture dish, spread 10,000 cells per well in 96-well plates, and spread 150,000 cells per well in 12-well plates, and culture overnight until the cells adhere to the wall.
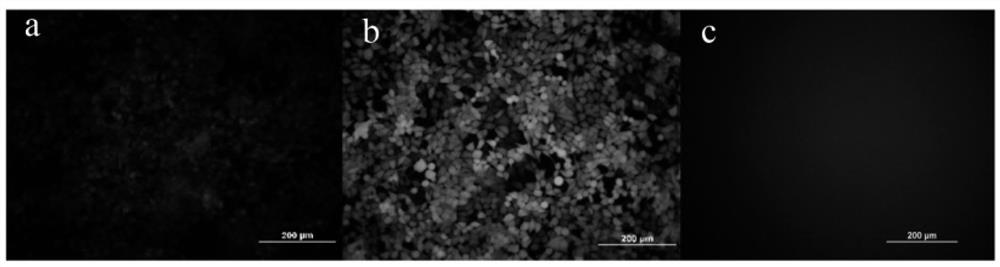
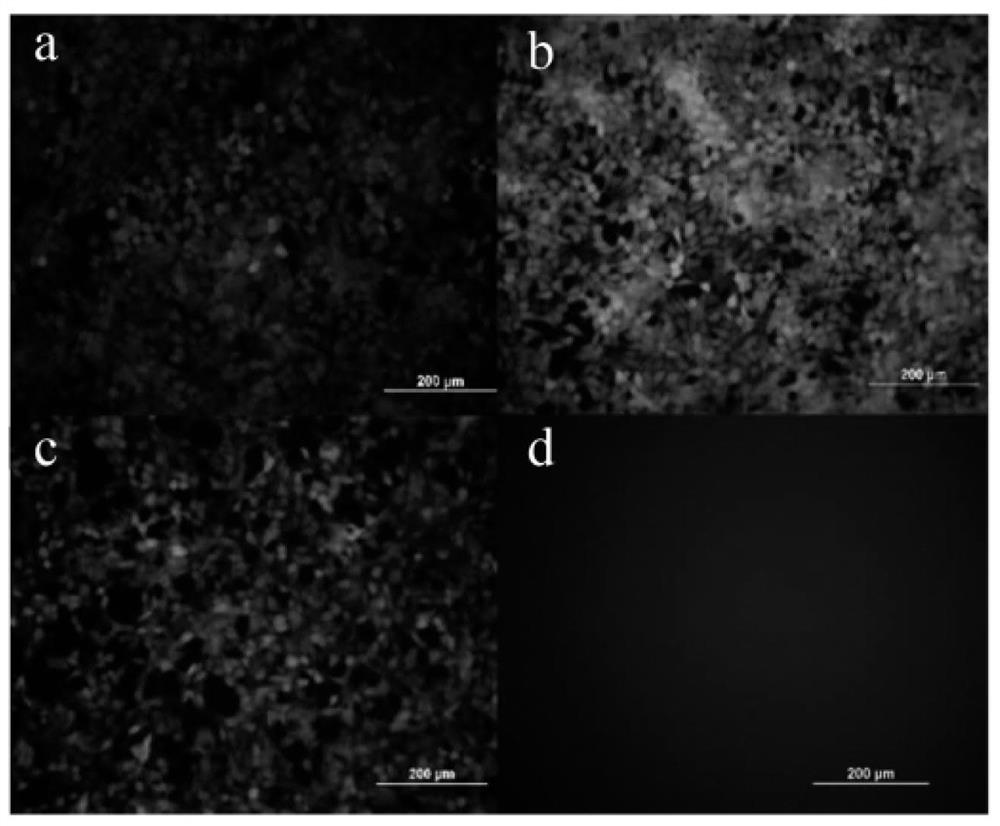
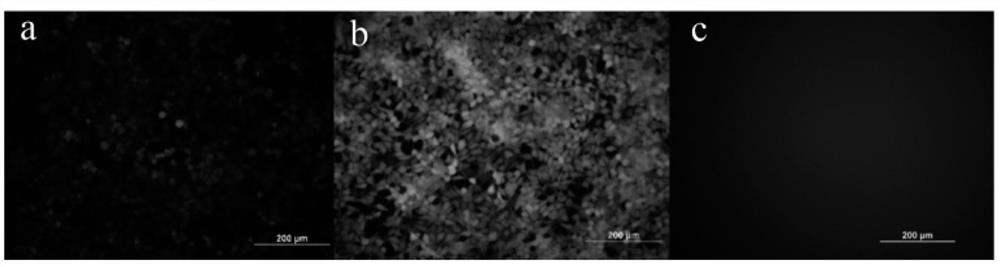
[0341] Cell transfection experiment: the LNP preparation containing 1.5 μg of eGFP-mRNA prepared in Example 2 (the cationic lipid in the carrier is YK-009) and the Lipofectamin2000 preparation of eGFP-mRNA were added to the cell culture medium of the 12-well plate, After continuing to culture for 24 hours, the transfection efficiency of different samples was investigated according to the fluorescence intensity by observing with a fluorescence microscope.
[0342] According to the experimental results, the preparation conditions of lipid nanopa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com