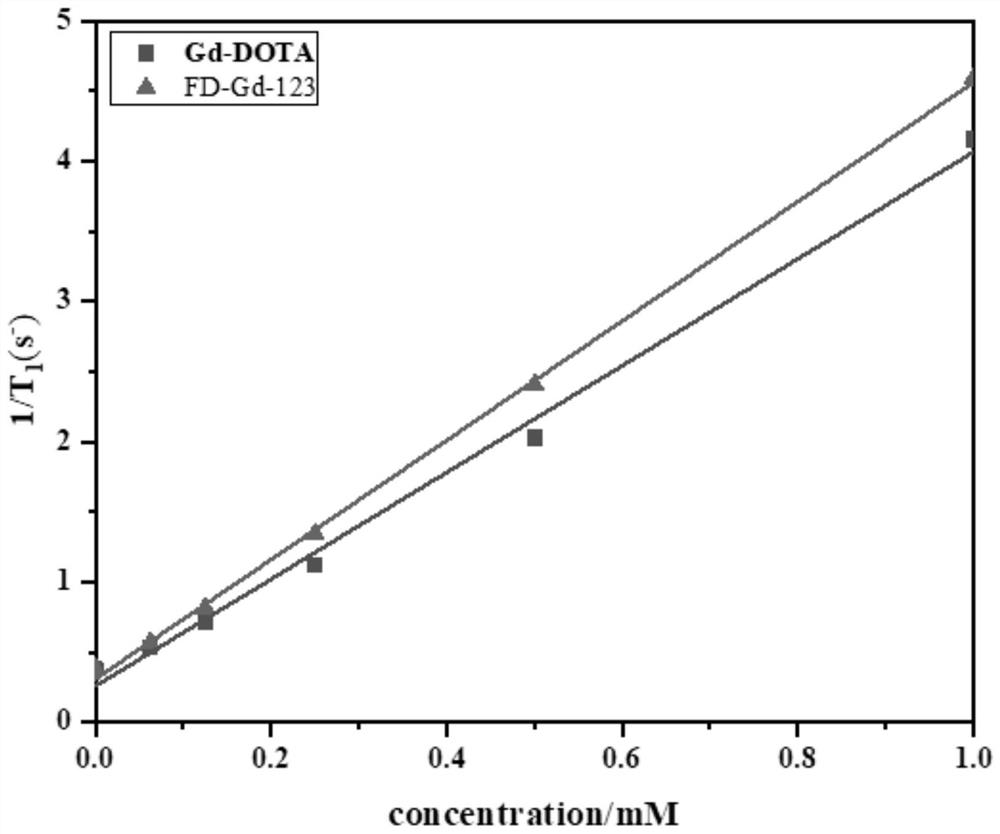
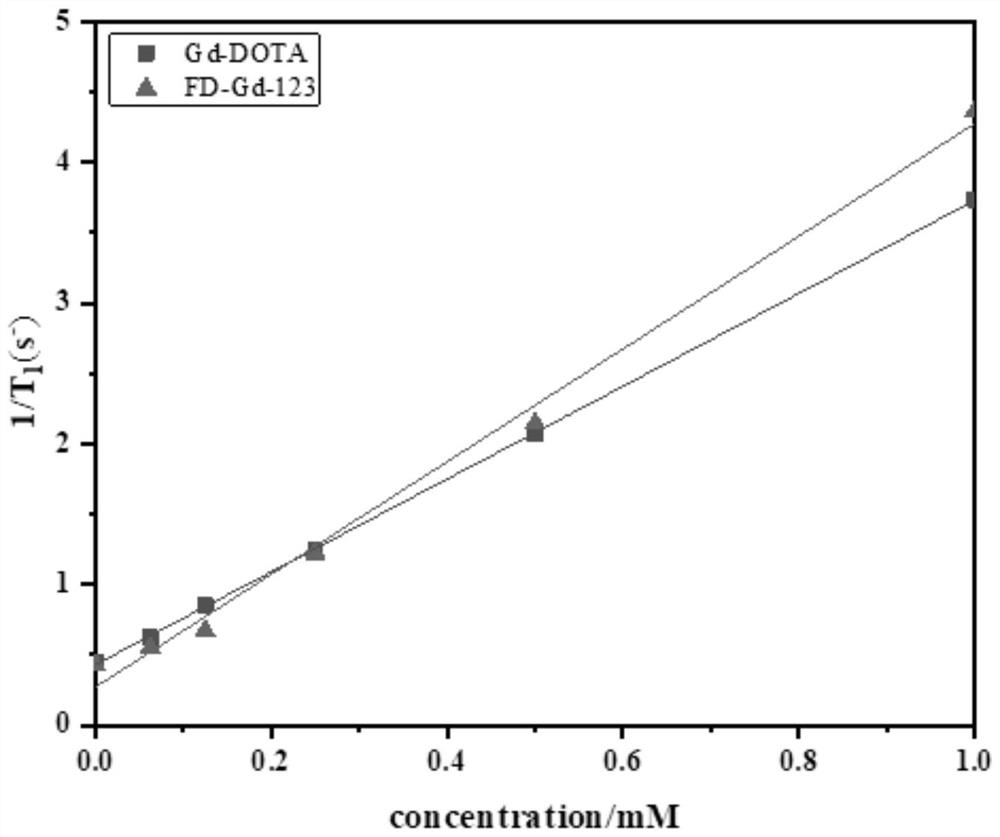
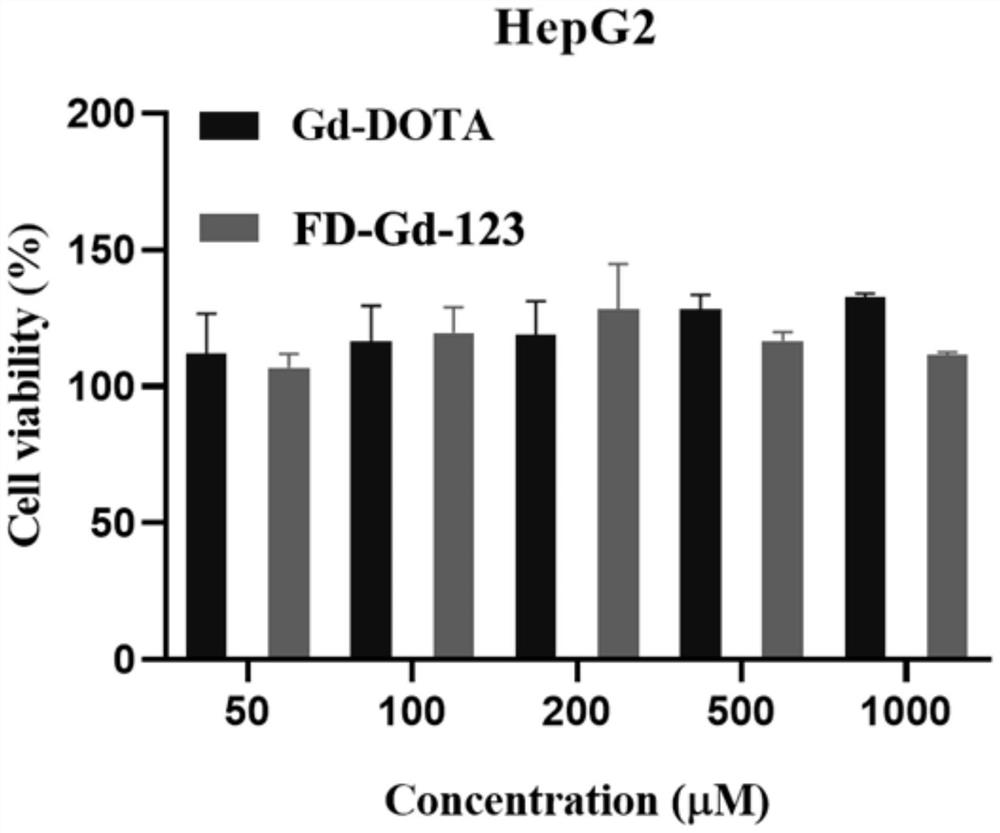
Gadolinium-based T1 magnetic resonance contrast agent FD-Gd-123 as well as preparation method and application thereof
A magnetic resonance contrast agent, fd-gd-123 technology, which is applied in the field of gadolinium-based T1 magnetic resonance contrast agent and magnetic resonance imaging angiography, and can solve the problem of low relaxation rate, high use dose, and superposition of safety risks of ionic chelate compounds. and other issues to achieve the effect of improving biosecurity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053]
[0054] Gd(NO 3 ) 3 6H 2 O (0.082g) and DO3A-S-pyridine-o-carboxylic acid (0.10g) were dissolved in 4ml methanol at a feed ratio of 1:1.1, and stirred. Adjust the pH to about 6 with 1M sodium hydroxide solution, react at room temperature for 0.5 h, and let stand overnight. After passing through a C-18 silica gel column and eluted with a gradient of 1% methanol / water, 0.06 g of white solid Gd-DO3A-S-pyridine-o-carboxylic acid I-S-1 was obtained. MS (ESI + ):m / z740.0011([M+Na] + ).
Embodiment 2
[0056]
[0057] Gd(NO 3 ) 3 6H 2 O (0.075g) and DO3A-S-pyridine p-carboxylic acid (0.10g) were dissolved in 18ml of water at a feed ratio of 1:1.2, and stirred. Adjust the pH to about 6 with 1M sodium hydroxide solution, react at 120°C for 24 h, and let stand overnight. C-18 silica gel column, 1% methanol / water gradient elution to obtain Gd-DO3A-S-pyridine p-carboxylic acid I-S-3 white solid 0.05g. MS (ESI + ):m / z655.0812([M+H] + ).
Embodiment 3
[0059]
[0060] Gd(AcO) 3 6H 2 O (0.041g) and DO3A-O-pyridine meta-carboxylic acid (0.05g) were dissolved in 2ml of water at a feed ratio of 1:1.1, and stirred. Adjust the pH to about 6 with 1M sodium hydroxide solution, react at room temperature for 24 hours, and let stand overnight. After passing through a C-18 silica gel column and eluted with a gradient of 1% methanol / water, 0.04 g of white solid Gd-DO3A-O-pyridine meta-carboxylic acid I-O-2 was obtained. MS (ESI + ):m / z639.1011([M+H] + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com