Method for producing hydrogen based on hydrolysis of Mg-Ni-Y alloy containing LPSO second phase
A hydrolysis hydrogen production and alloy technology, applied in the field of hydrogen production, can solve the problems of increasing the cost of hydrogen production, reducing the hydrogen production of magnesium alloys, and environmental pollution of metal ions, so as to reduce the cost of hydrogen production, obtain seawater easily, and simplify hydrogen production The effect of craft
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: A method for hydrogen production based on the hydrolysis of Mg-Ni-Y alloy containing LPSO second phase, the specific steps are as follows:
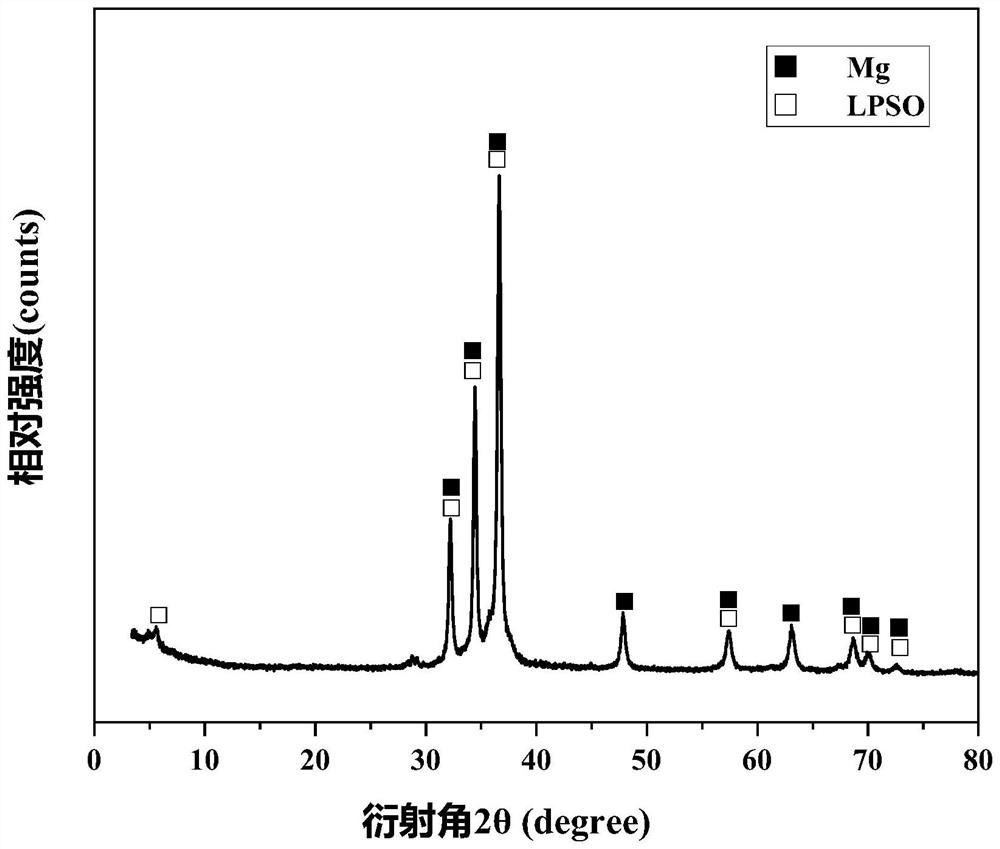
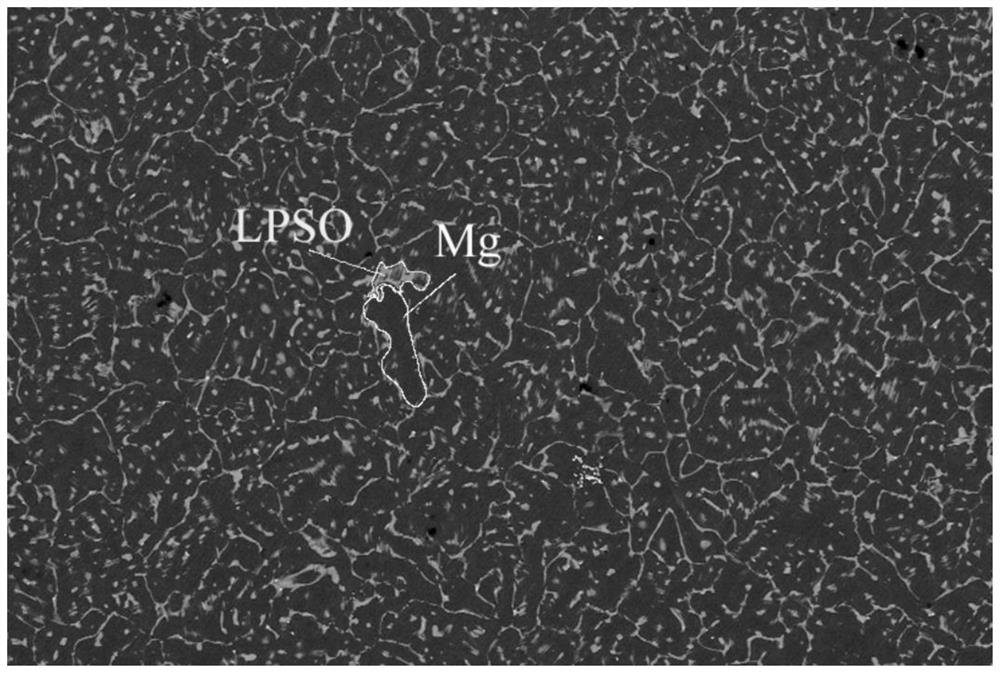
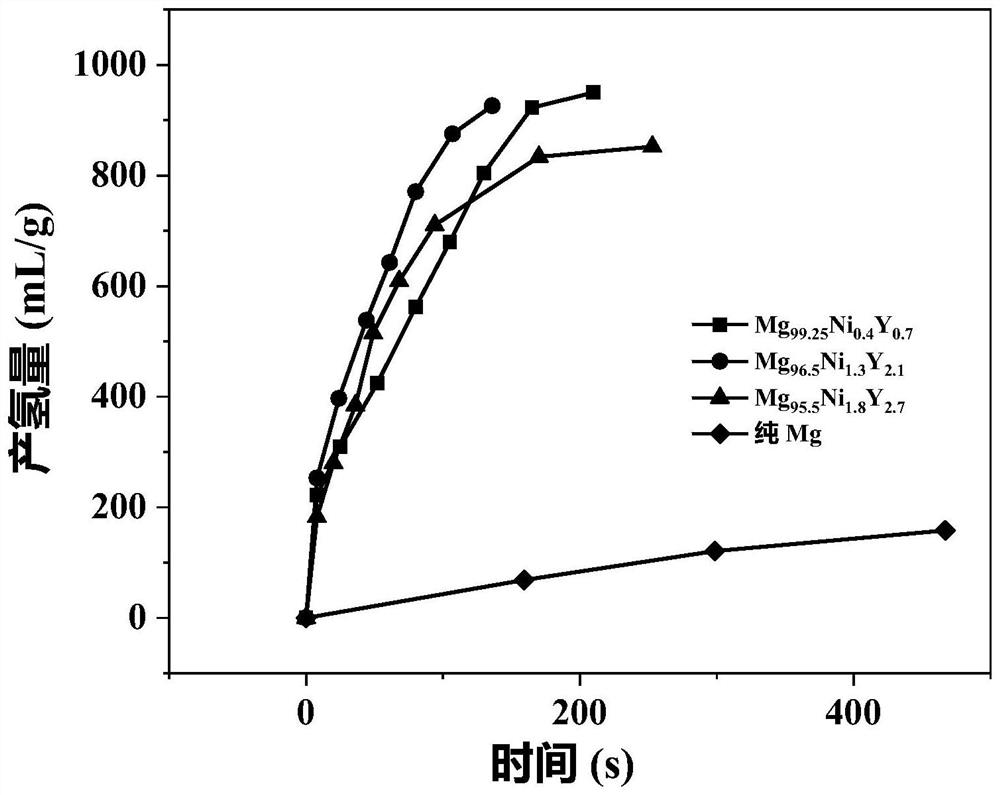
[0023] At room temperature, 30 mg of Mg-Ni-Y alloy powder containing LPSO second phase was added to 10 mL of simulated seawater (that is, the solid-liquid ratio g:L of Mg-Ni-Y alloy powder containing LPSO second phase to seawater was 3 : 1) The hydrolysis reaction produces hydrogen, and the hydrogen is collected by the drainage method; the chemical composition of the Mg-Ni-Y alloy containing the second phase of LPSO is Mg 96.5 Ni 1.3 Y 2.1 , the phase of Mg-Ni-Y alloy containing LPSO second phase includes Mg phase and long-period stacking structure phase, that is, LPSO phase, and the volume ratio of LPSO phase is 16.8%; the mass concentration of NaCl in simulated seawater is 3.5%;
[0024] The preparation method of the Mg-Ni-Y alloy powder containing LPSO second phase comprises the following specific steps:
[0025...
Embodiment 2
[0030] Embodiment 2: A method for hydrogen production based on the hydrolysis of Mg-Ni-Y alloy containing LPSO second phase, the specific steps are as follows:
[0031] At room temperature, 30 mg of Mg-Ni-Y alloy powder containing LPSO second phase was added to 10 mL of simulated seawater (that is, the solid-liquid ratio g:L of Mg-Ni-Y alloy powder containing LPSO second phase to seawater was 3 : 1) The hydrolysis reaction produces hydrogen, and the hydrogen is collected by the drainage method; the chemical composition of the Mg-Ni-Y alloy containing the second phase of LPSO is Mg 99.25 Ni 0.25 Y0.5 , the phase of the Mg-Ni-Y alloy containing the second LPSO phase includes the Mg phase and the long-period stacking structure phase, that is, the LPSO phase, and the volume ratio of the LPSO phase is 4.9%; the mass concentration of NaCl in the simulated seawater is 3.5%;
[0032] The preparation method of the Mg-Ni-Y alloy powder containing LPSO second phase comprises the followi...
Embodiment 3
[0037] Embodiment 3: A method for hydrogen production based on the hydrolysis of Mg-Ni-Y alloy containing LPSO second phase, the specific steps are as follows:
[0038] At room temperature, 30 mg of Mg-Ni-Y alloy powder containing LPSO second phase was added to 10 mL of simulated seawater (that is, the solid-liquid ratio g:L of Mg-Ni-Y alloy powder containing LPSO second phase to seawater was 3 : 1) The hydrolysis reaction produces hydrogen, and the hydrogen is collected by the drainage method; the chemical composition of the Mg-Ni-Y alloy containing the second phase of LPSO is Mg 95.5 Ni 1.8 Y 2.7 , the phase of the Mg-Ni-Y alloy containing the LPSO second phase includes the Mg phase and the long-period stacking structure phase, that is, the LPSO phase, and the volume ratio of the LPSO phase is 26%; the mass concentration of NaCl in the simulated seawater is 3.5%;
[0039] The preparation method of the Mg-Ni-Y alloy powder containing LPSO second phase comprises the followin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com