Precision medical methods for cancer immunotherapy
A technology for immunotherapy and cancer, applied in the field of precision medicine for cancer immunotherapy, which can solve the problems of decreased neutrophil/lymphocyte ratio and increased number
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
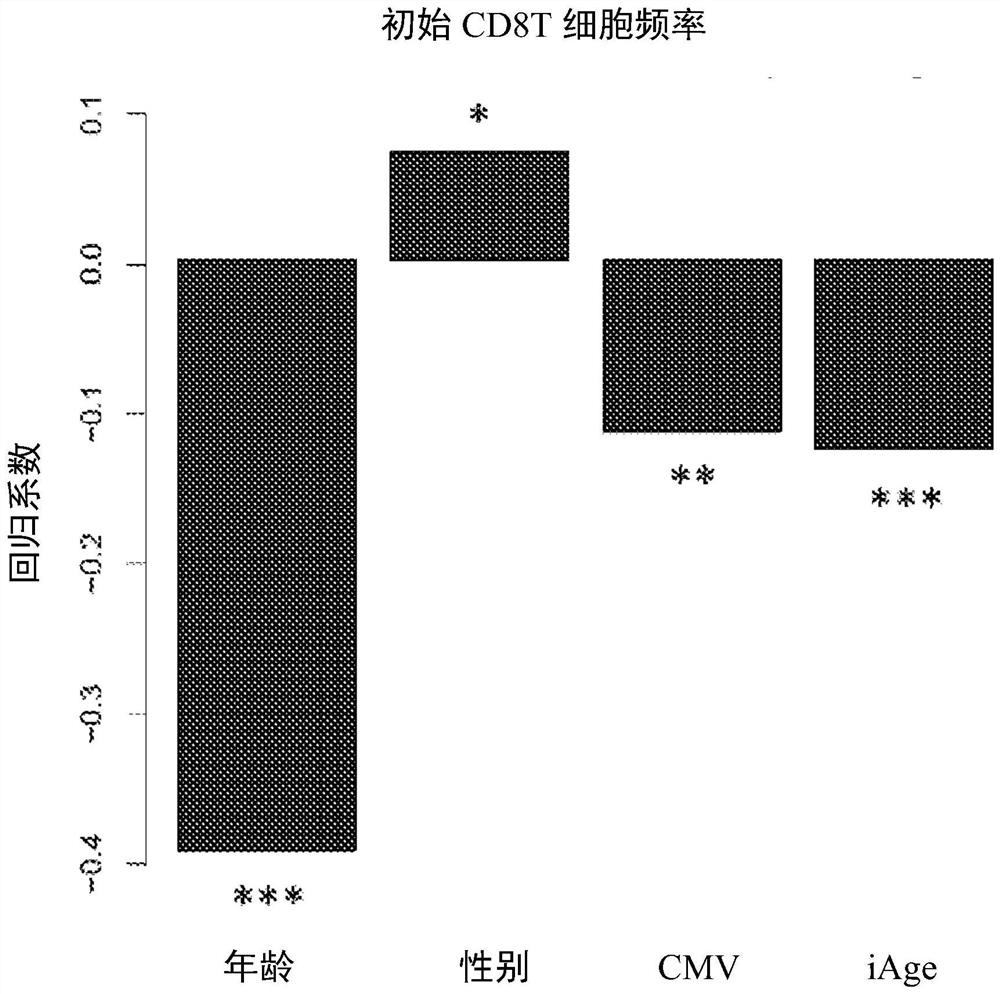
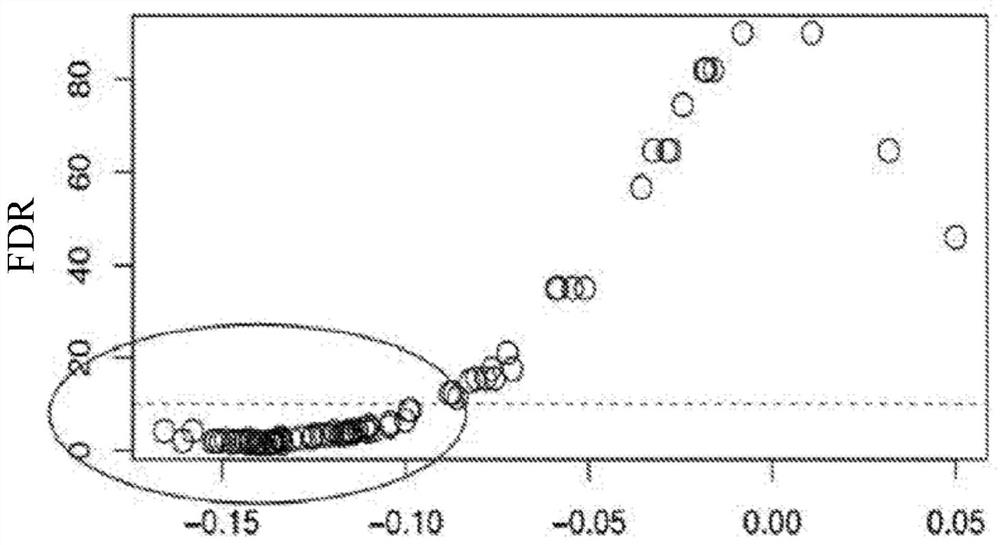
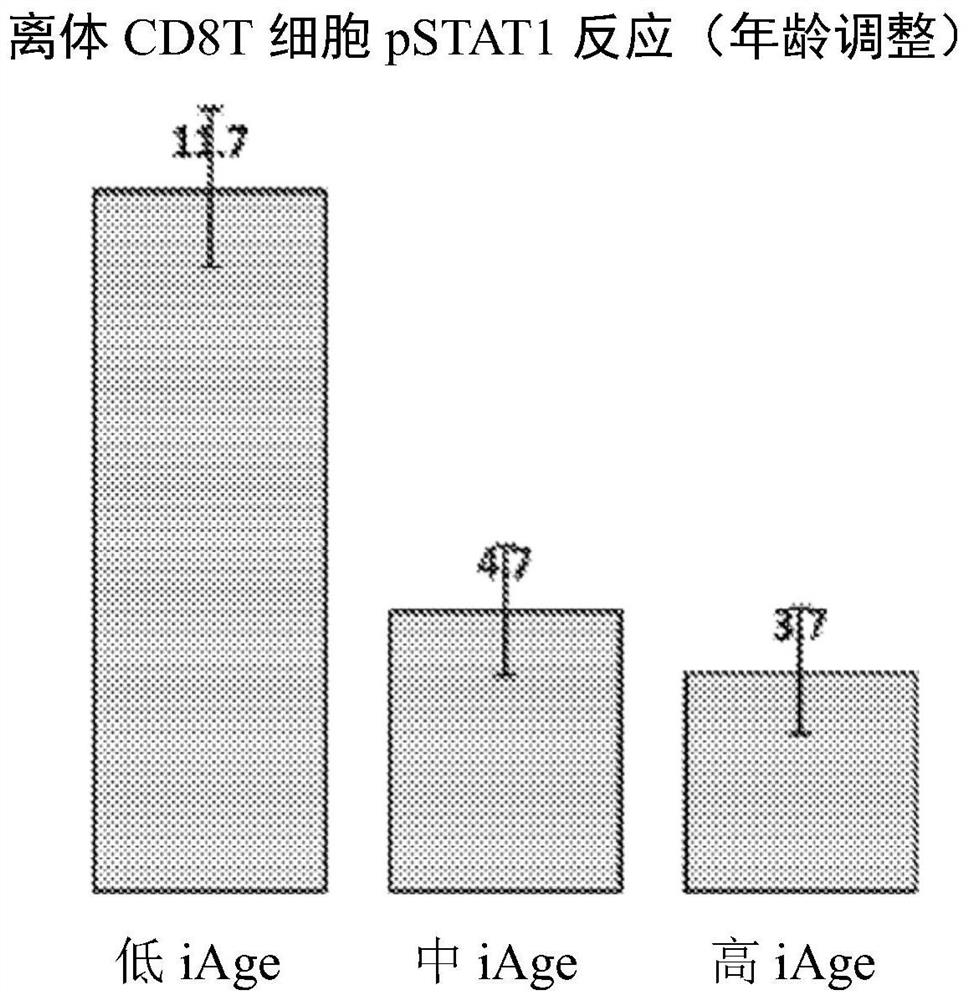
[0090] Example 1: iAge is associated with naïve CD8(+) T cells and ex vivo Jak-STAT signaling responses to stimulation
[0091] Frequency of circulating naive CD8(+) T cells decreases with high iAge (A), which predicts poor response to stimulated ex vivo Jak-STAT signaling (B and C). A total of 96 cytokine-cell-STAT combinations were analyzed with respect to subjects' iAge. These include eight cell types: B cells, CD4(+) T cells (and their CD45(+) and (-) subsets), CD8(+) T cells (and their CD45(+) and (-) ) and monocytes; four cytokines: interleukin-6 (IL-6), IL-10, IL-21, and interferon-α; and three STAT proteins (STAT1, 3, and 5). Figure 1B : Volcano plot, results of multiple regression analysis with permutation test estimating the false discovery rate (Benjamini-Hochberg FDR) (y-axis) as a function of regression coefficients obtained for iAge, after adjusting for age, sex, and CMV status. Figure 1C : Normalized ex vivo CD8(+) T cell phospho-STAT-1 response to interle...
example 2
[0093] Example 2: Stratification of cancer patients using iAge and CRS
[0094] Before immunotherapy treatment, a blood sample is obtained from the patient. Serum and immune cells were isolated by standard methods. Serum samples were used to measure protein concentrations to determine inflammatory age (iAge); cells were stimulated ex vivo with cytokines to measure phosphorylation of intracellular signal transducers and activators of transcription (STAT) proteins, resulting in a cytokine response score ( CRS). iAge and CRS can independently predict patient response to immunotherapy treatment. figure 2 A flowchart of the process is shown.
[0095] iAge and CRS can be used to stratify cancer patients into immunotherapy responders and non-responders prior to treatment.
example 3
[0096] Example 3: Using iAge to Stratify Cancer Patients
[0097]iAge can be used to classify cancer patients into responders and non-responders to immunotherapy treatment (A), and derive iAge individual inflammatory protein signatures / signatures (barcodes), which can be fed into the iAge personalized recommendation engine to create targeted Individualized initial therapy that lowers iAge to inform medical decision-making, thereby converting non-responders to responders (suitable for immunotherapy) (B). image 3 A flowchart of the process is shown.
[0098] iAge is being used to stratify patients for cancer immunotherapy and to help convert non-responders to responders to immunotherapy.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com