Ureido compound or pharmaceutical salt thereof as well as preparation method and application thereof
A compound and medicinal salt technology, applied in the field of medicinal chemistry, can solve the problems of limiting the clinical use of microtubule-targeted drugs, multidrug resistance, etc., and achieve the effect of improving anti-tumor effect and enhancing proliferation inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
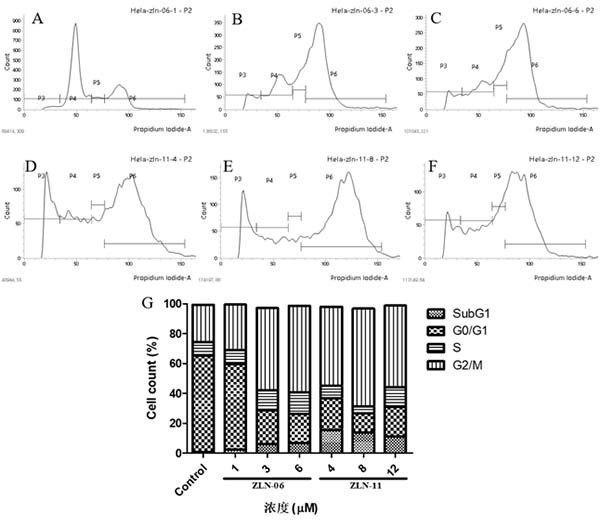
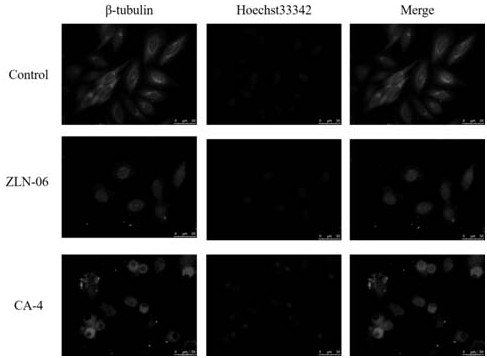
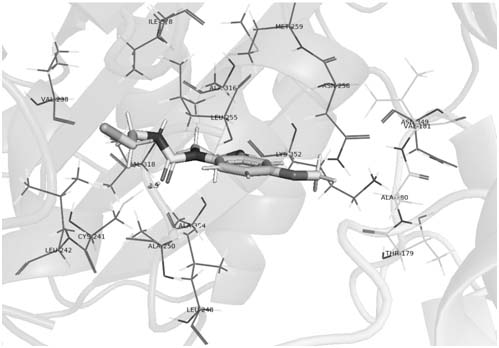
Image
Examples
Embodiment 1
[0046] Synthesis of 6-methoxy-N-(2-methoxyphenyl)-3,4-dihydroquinoxaline-1(2H)-carboxamide (I-1)
[0047]
[0048] Synthesis of N-Benzyl-5-methoxy-2-nitroaniline (III-1)
[0049] Benzylamine (2.07g, 19.28mmol) was dissolved in acetonitrile (40mL), and 3-fluoro-4-nitroanisole (Ⅱ-1) (3.00g, 17.50mmol) and anhydrous potassium carbonate (4.84g , 35.02mmol), reflux and stir at 85°C. After 90 minutes, TLC (developing agent PE:EA=5:1) monitored whether the reaction was complete. After the raw materials were completely reacted, stop heating, cool to room temperature, and filter with suction. The filtrate was washed with water (20mL×3), saturated sodium chloride solution (20mL ×3) Wash and dry overnight with anhydrous sodium sulfate. After suction filtration, the filtrate was concentrated under reduced pressure to obtain 3.92 g of a yellow solid, with a yield of 86.6%. The product was directly sent to the next step without further purification. 1H NMR (300MHz, DMSO-d6) δ8.87(t, ...
Embodiment 2
[0061] Synthesis of 6-methoxy-N-(4-methoxyphenyl)-3,4-dihydroquinoxaline-1(2H)-carboxamide (I-2)
[0062]
[0063] Synthesis of 4-Benzyl-6-methoxy-N-(4-methoxyphenyl)-3,4-dihydroquinoxaline-1(2H)-carboxamide (VIII-2)
[0064] Using VI-1 (300mg, 1.18mmol) and 4-methoxyisocyanate (VII-2) (188mg, 1.42mmol) as raw materials, the operation process was the same as that of intermediate VIII-1 to obtain 327mg of white solid with a yield of 68.7%. 1H NMR (300MHz, CDCl3) δ7.36-7.26 (m, 5H), 7.21 (d, J = 7.1Hz, 2H), 7.13 (d, J = 8.2Hz, 1H), 7.06 (s, 1H), 6.83 (d, J=8.9Hz, 2H), 6.31-6.21(m, 2H), 4.54(s, 2H), 3.90(t, J=4.8Hz, 2H), 3.77(s, 3H), 3.70(s, 3H), 3.46(t, J=5.2Hz, 2H).
[0065] Synthesis of 6-methoxy-N-(4-methoxyphenyl)-3,4-dihydroquinoxaline-1(2H)-carboxamide (I-2)
[0066]Using VIII-2 (300 mg, 0.74 mmol) as the starting material, the operation process was the same as that of the target compound I-1 to obtain 162 mg of white solid with a yield of 69.9%. 1H NMR (300MHz, CDC...
Embodiment 3
[0068] Synthesis of 6-methoxy-N-(4-(trifluoromethoxy)phenyl)-3,4-dihydroquinoxaline-1(2H)-carboxamide (I-3)
[0069]
[0070] 4-Benzyl-6-methoxy-N-(4-(trifluoromethoxy)phenyl)-3,4-dihydroquinoxaline-1(2H)-carboxamide (VIII-3) synthesis
[0071] Using VI-1 (300mg, 1.18mmol) and 4-trifluoromethoxyisocyanate (VII-3) (262mg, 1.29mmol) as raw materials, the operation process was the same as that of intermediate VIII-1 to obtain 399mg of white solid, yield 74.0 %. 1H NMR (300MHz, CDCl3) δ7.45-7.39(m,2H),7.36-7.27(m,3H),7.25-7.22(m,2H),7.20(s,1H),7.17-7.08(m,3H ),6.30-6.24(m,2H),4.54(s,2H),3.91(t,J=5.3Hz,2H),3.71(s,3H),3.47(t,J=5.3Hz,2H).
[0072] Synthesis of 6-methoxy-N-(4-(trifluoromethoxy)phenyl)-3,4-dihydroquinoxaline-1(2H)-carboxamide (I-3)
[0073] Using VII-3 (350 mg, 0.77 mmol) as the starting material, the operation process was the same as that of the target compound I-1 to obtain 170 mg of a white solid with a yield of 60.3%. 1H NMR (300MHz, CDCl3) δ7.43-7.36(m, 2H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com