Preparation method and application of a copper-catalyzed tetrahydropyrroloquinoline derivative
A technology of tetrahydropyrrole and derivatives, which can be used in organic chemistry, drug combination, antitumor drugs, etc., can solve the problems of long reaction time, limited substrate range, expensive catalyst, etc., and achieves low cost, high yield and time. quick effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1 preparation of aminoalkyne compound
[0037]
[0038] Add 3-butyn-1-ol (5.0 g, 72 mmol), imidazole (6.8 g, 100 mmol) into a one-necked bottle. Add dichloromethane to dissolve. Add tert-butyldimethylsilyl chloride (12.9 g, 86 mmol) under ice-bath conditions, stir at room temperature for 30 minutes, and check the completion of the reaction by thin-layer chromatography, extract with dichloromethane, wash with water, and dry. Column chromatography (petroleum ether / ethyl acetate=50:1) yielded 13.2 g of a colorless oily liquid.
[0039] Weigh the TBS (tert-butyldimethylsilyl)-protected acetylenic alcohol (15mmol) obtained in the previous step and add it to an anhydrous three-neck reaction flask, add anhydrous tetrahydrofuran (20mL) under the protection of argon, and put the reaction at -78°C With stirring at ambient, n-butyllithium (2.5M, 8 mL) was slowly added thereto. Stir at -78°C for 1 hour, then add iodomethane (45 mmol), and react overnight at room te...
Embodiment 2
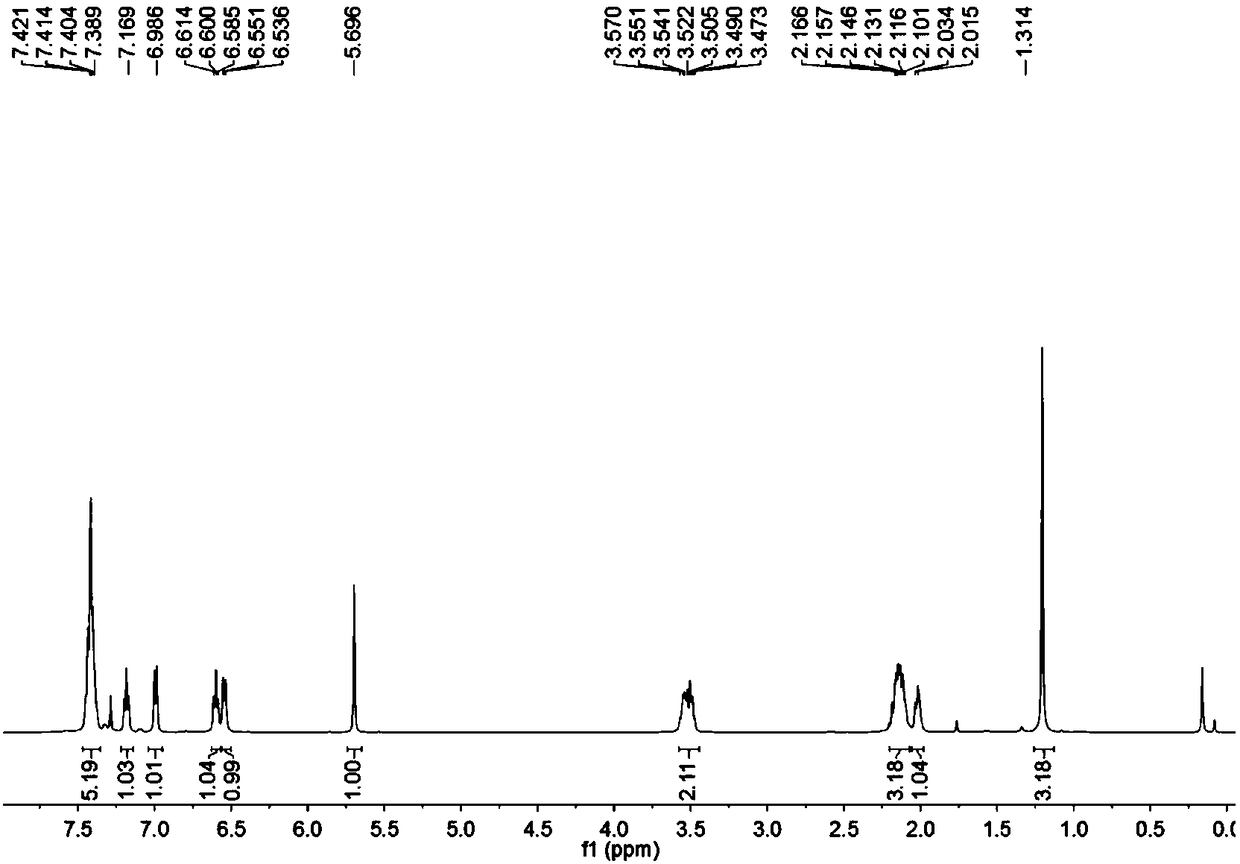
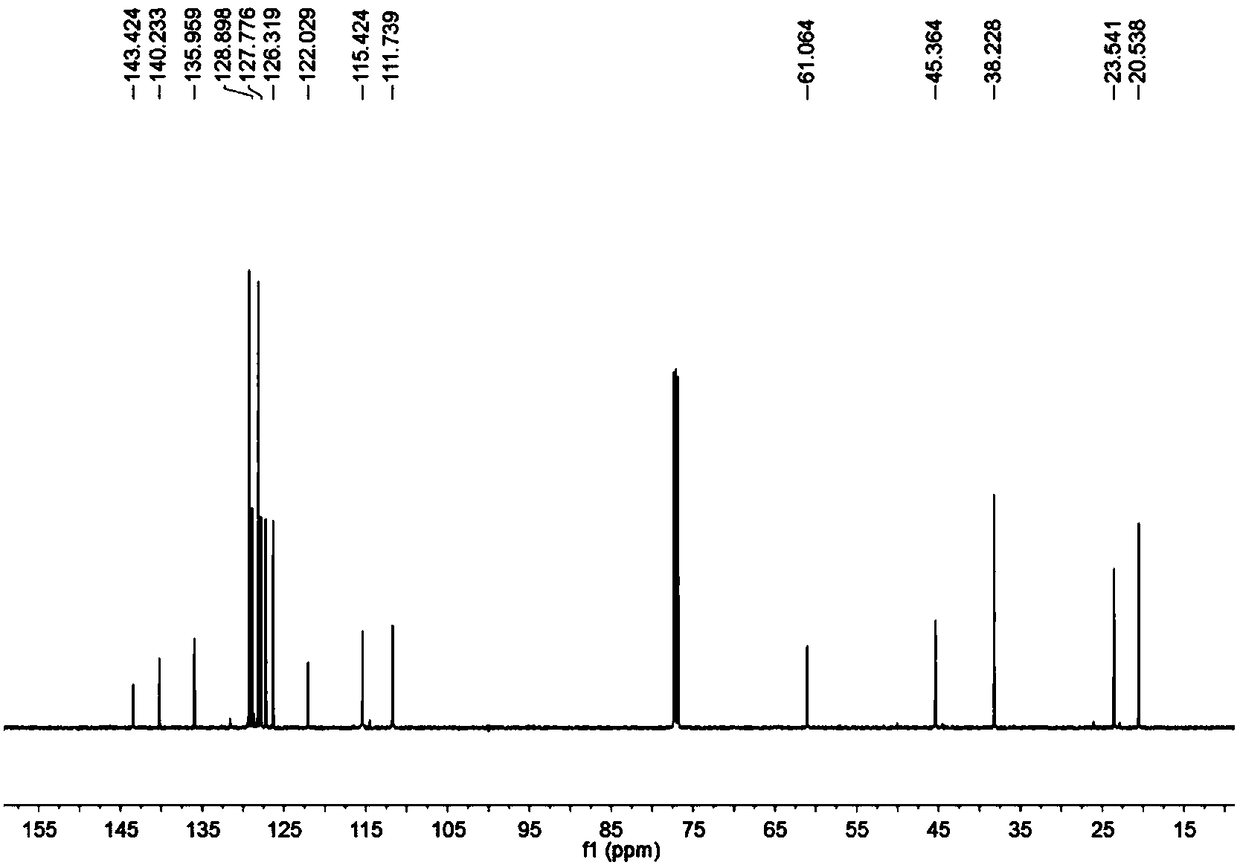
[0043] To a microwave tube equipped with a magneton was added cuprous chloride (2 mg, 0.02 mmol) under nitrogen, followed by 5 mL of anhydrous DMF by syringe, and finally, aminoalkyne (31.8 mg, 0.2 mmol) was added under nitrogen , phenylacetylene (61.2mg, 0.6mmol) under microwave irradiation, reacted at 150°C for 30 minutes, after TLC detected that the reaction was complete, added ethyl acetate, water layer, washed 3 times with ethyl acetate, washed 5 times with water, combined the organic layer, Dry over anhydrous sodium sulfate. Pass through the column (eluent: petroleum ether / ethyl acetate=50 / 1) to obtain 47 mg of a yellow solid. of the resulting product 1 HNMR spectrum, 13 CNMR spectrum, see figure 1 , figure 2 .
[0044]
[0045] The physical properties and spectral data of the product are as follows: yellow solid; 1 H NMR (500MHz, CDCl 3 )δ7.50–7.33(m,5H),7.16(t,J=7.5Hz,1H),6.97(d,J=7.5Hz,1H),6.57(t,J=7.5Hz,1H),6.52( d,J=7.5Hz,1H),5.67(s,1H),3.59–3.42(m,2H),2...
Embodiment 3
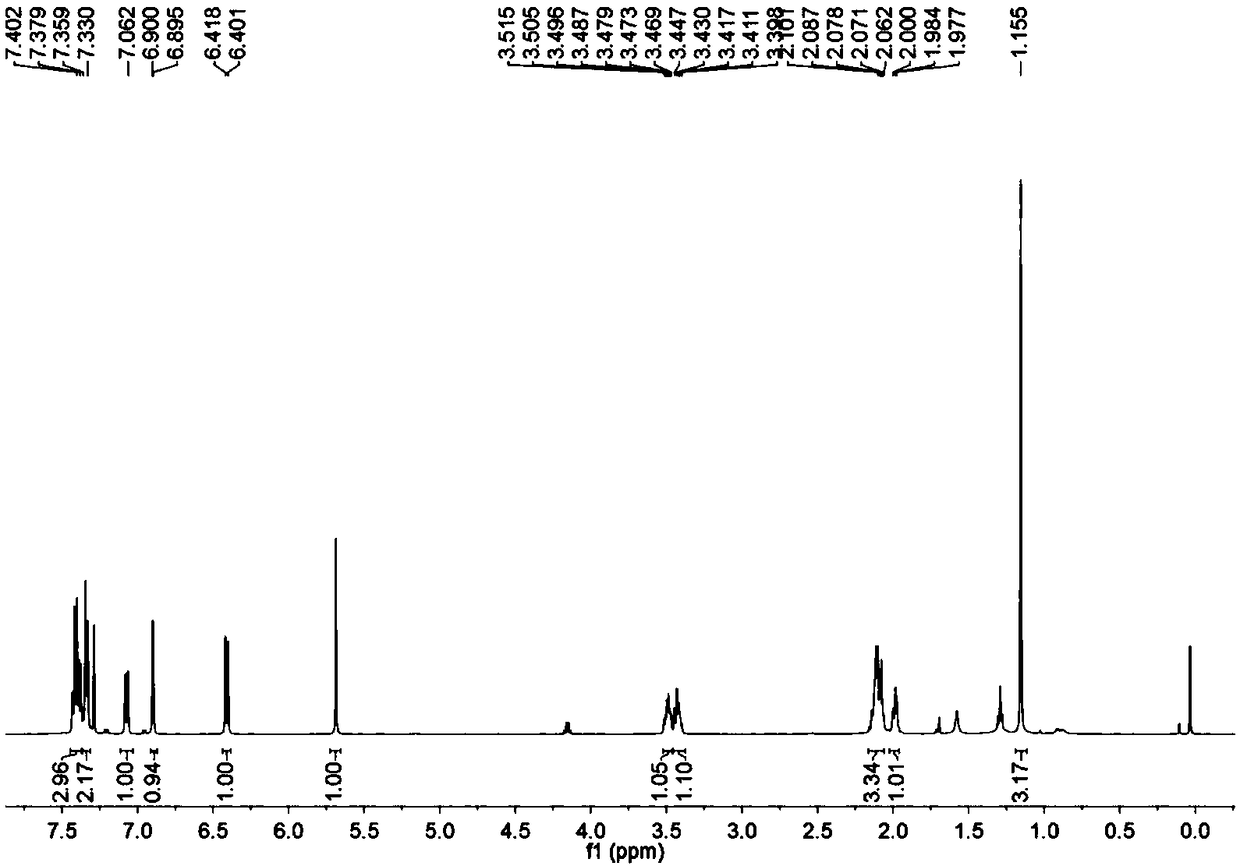
[0047] To a microwave tube equipped with a magnet was added cuprous chloride (2mg, 0.02mmol) under nitrogen atmosphere, then 5mL of anhydrous DMF was added by syringe, and finally, aminoalkyne (38.6mg, 0.2mmol) was added under nitrogen atmosphere , phenylacetylene (61.2mg, 0.6mmol) under microwave irradiation, reacted at 150°C for 30 minutes, after TLC (thin layer chromatography) detected that the reaction was complete, add ethyl acetate, water layer, wash with ethyl acetate 3 times, wash with water 5 times, the combined organic layers were dried over anhydrous sodium sulfate. Pass through the column (eluent: petroleum ether / ethyl acetate=50 / 1) to obtain 42 mg of yellow solid. of the resulting product 1 HNMR spectrum, 13 CNMR spectrum, see image 3 , Figure 4 .
[0048]
[0049] The physical properties and spectral data of the product are as follows: yellow solid; 1 H NMR (500MHz, CDCl 3 )δ7.44–7.37(m,3H),7.35–7.31(m,2H),7.07(dd,J=8.5,2.5Hz,1H),6.90(d,J=2.5Hz,1H),6....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com