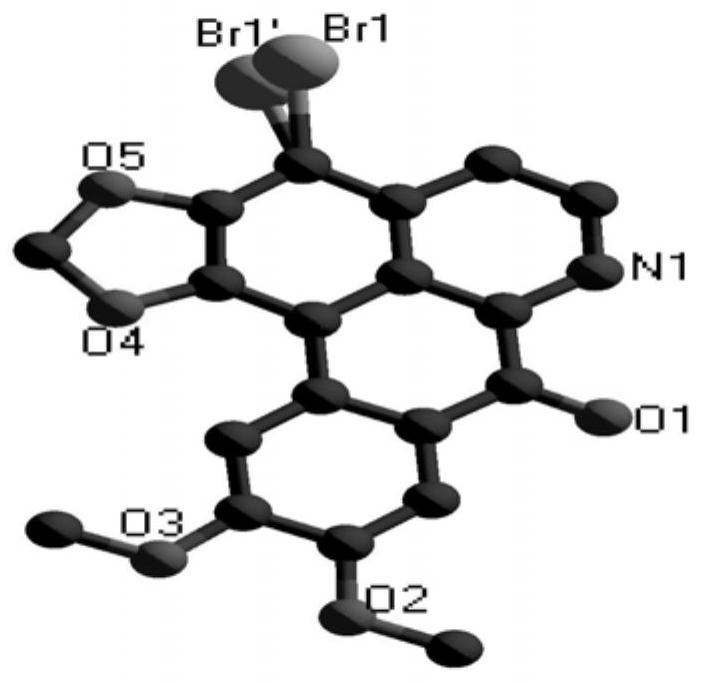
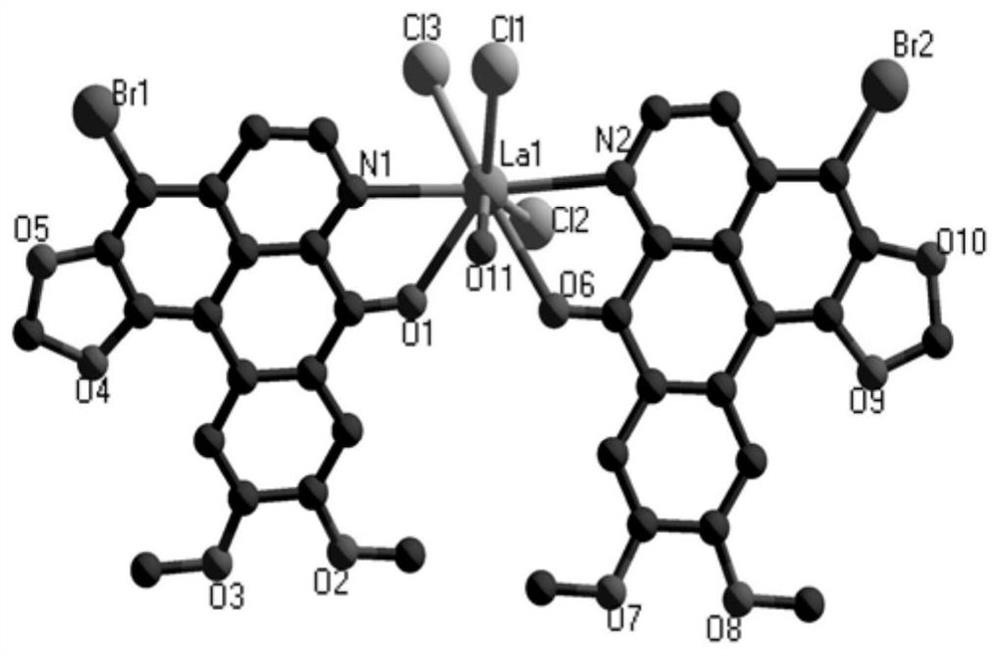
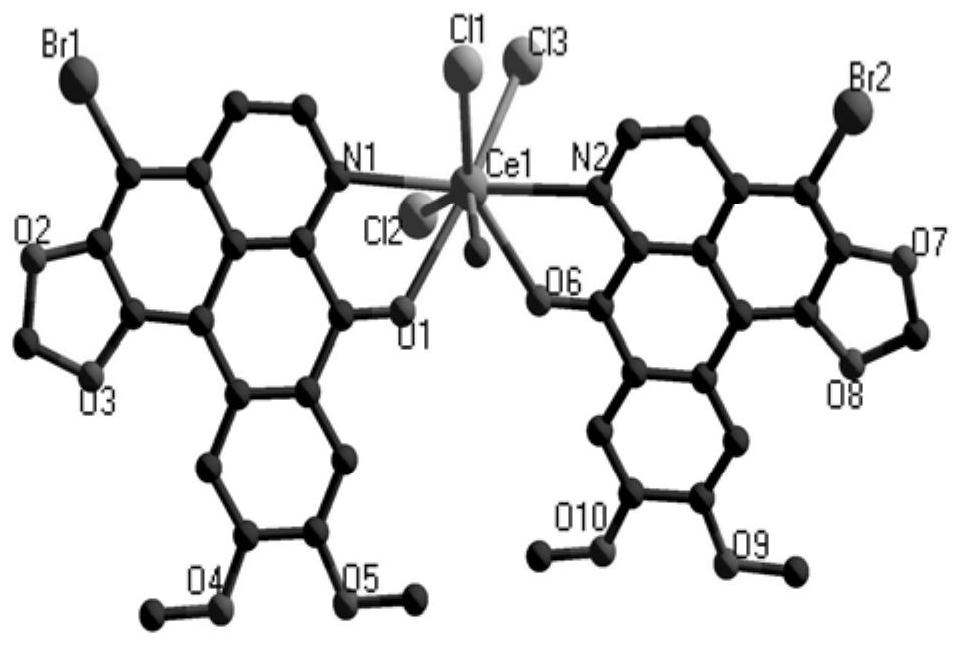
Rare earth complex with brominated oxybicuculline as a ligand, its synthesis method and application
A synthesis method and compound technology, applied in the field of rare earth complexes and their synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1: the synthesis of brominated oxidized bicuculline i.e. Br-DCO
[0059] Synthesized according to the aforementioned synthetic route, specifically comprising the following steps:
[0060] 1) Synthesis of compound (2):
[0061] Dissolve 72g of 3,4-dimethoxyphenylacetic acid in 600mL of glacial acetic acid, stir at room temperature for 1h, then add 7.2g of bromine in glacial acetic acid (60mL), continue to react for 2h, then add 200mL of ice water, white A precipitate was formed, filtered, and the filter cake was recrystallized with methanol to obtain 96 g of compound (2), with a yield of about 95%.
[0062] Compound (2) is a white solid, ESI-MS m / z 273.02[(2)-H] - , 13 C-NMR (500MHz, DMSO) δ: 41.0816 (C-2), 56.2538 (C-5), 56.4330 (C-6), 114.9042 (C-9), 115.7982 (C-3), 115.9616 (C-8 ), 127.4302(C-10), 148.5825(C-4), 148.9687(C-8), 172.0429(C-1), 1 H-NMR (500MHz, DMSO) δ: 3.6021 (2H, S, H-2), 3.7120 (3H, S, H-5), 3.7346 (3H, S, H-6), 6.9880 (1H, S, H -3), ...
Embodiment 2
[0093] Embodiment 2: the synthesis of Br-DCO
[0094] Repeat Example 1, the difference is:
[0095] In steps 3)-5), the first organic solvent involved is changed to ethanol;
[0096] In step 4), reducing agent is changed into lithium borohydride;
[0097] In step 5), adjust the pH=8 of the system, and use chloroform as the extractant instead;
[0098] In step 6), adjust the pH of the system to 10, change the amount of palladium acetate to 3% of the mass of compound (6), change the second organic solvent to DMAC, and use dichloromethane as the extractant;
[0099] In step 7), adjust the pH=9 of the system;
[0100] In step 8), the first organic solvent involved is changed to ethyl acetate, and the extractant is changed to ethyl acetate;
[0101] In step 9), the amount of manganese acetate (Ⅲ) is changed to 5 times of the mass of compound (9), and the eluent when eluted on the silica gel column is composed of dichloromethane and methanol in a volume ratio of 25-30:1 mixed s...
Embodiment 3
[0103] Embodiment 3: the synthesis of Br-DCO
[0104] Repeat Example 1, the difference is:
[0105] In step 3), the first organic solvent involved is changed to n-butanol;
[0106] In step 4), the first organic solvent involved is changed to n-propanol, and the reducing agent is changed to potassium borohydride;
[0107] In step 5), the first organic solvent involved is changed to methanol to adjust the pH of the system to 10;
[0108] In step 6), adjust the pH of the system to 10, change the amount of palladium acetate to 2% of the mass of compound (6), change the second organic solvent to DMAC, and use ethyl acetate as the extractant;
[0109] In step 7), adjust the pH of the system to 8.5;
[0110] In step 8), the first organic solvent involved is changed to methanol, and the extractant is changed to dichloromethane;
[0111] In step 9), the amount of manganese acetate (Ⅲ) is changed to 4 times of the mass of compound (9), and the eluent when eluted on the silica gel co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com