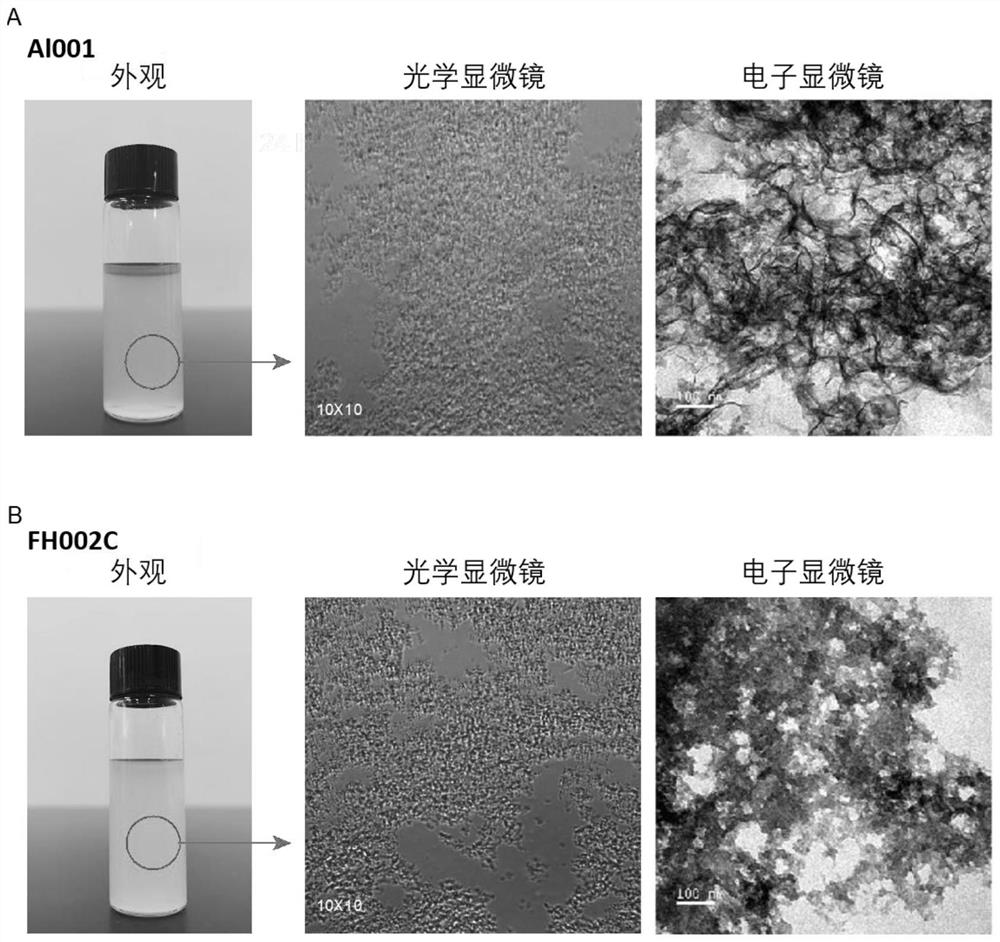
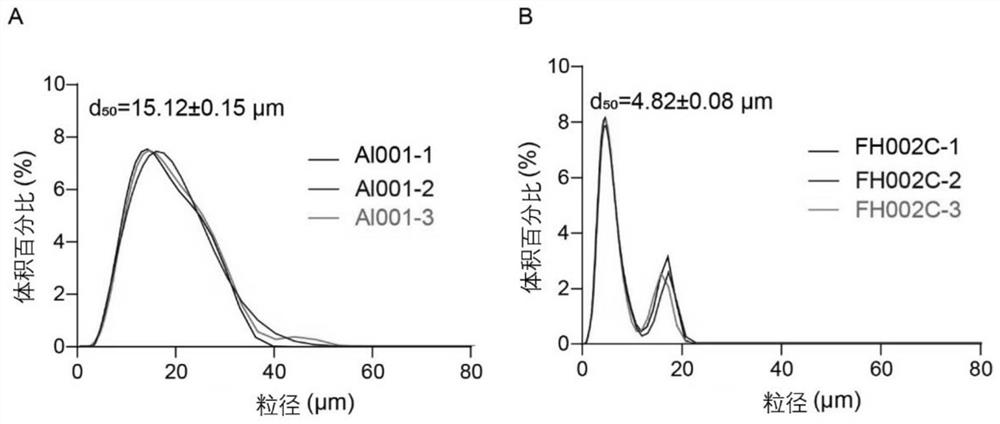
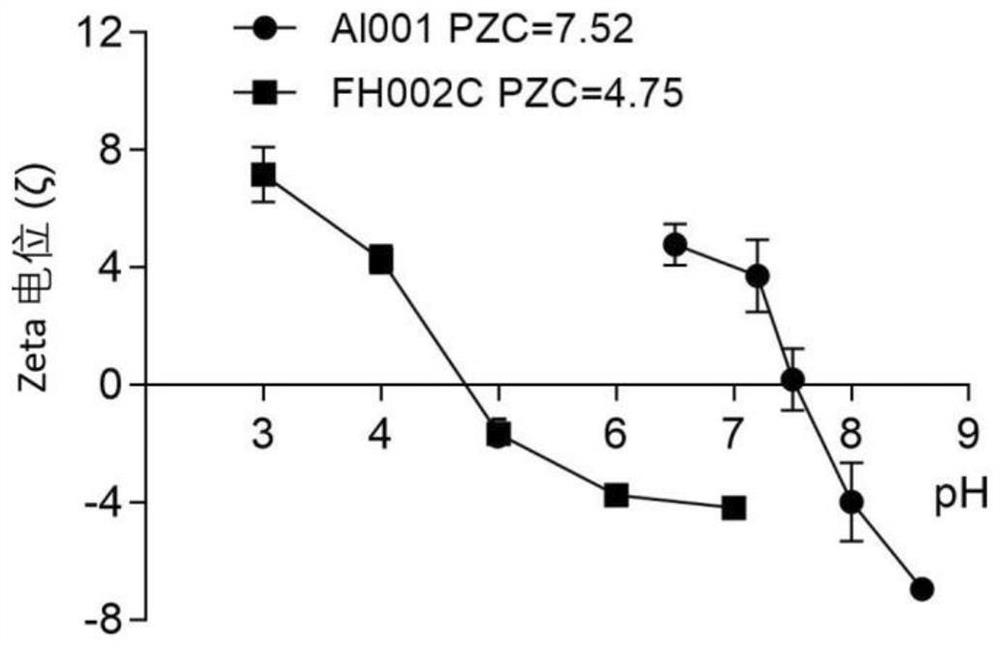
Adjuvant containing risedronate zinc aluminum and application thereof
A technology of zinc risedronate and risedronate, which is applied to medical preparations containing active ingredients, organic chemistry, antiviral agents, etc., can solve the problems of weak immunogenicity, etc. medicinal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0138] The reagents used in the preparation example are as follows:
[0139] Risedronate Sodium (C 7 h 10 NNaO 7 P 2 ), purchased from Hunan Huateng Pharmaceutical Co., Ltd.;
[0140] Anhydrous Zinc Chloride (ZnCl 2 ), purchased from Xilong Chemical Industry;
[0141] Aluminum Chloride Hexahydrate (AICl 3 ·6H 2 O), purchased from Xilong Chemical Industry;
[0142] Disodium hydrogen phosphate dodecahydrate (Na 2 HPO 4 12H 2 O), purchased from Xilong Chemical Industry;
[0143] Sodium hydroxide (NaOH), purchased from Xilong Chemical Industry
preparation example 1
[0144] Preparation Example 1: Preparation of Risedronate Zinc Aluminum Adjuvant (FH002C)
[0145] (1) Solution preparation:
[0146] According to the Zn / Al / risedronic acid molar concentration ratio of 1:0.1:0.22, prepare 0.5L (47mM zinc chloride+4.7mM aluminum chloride) solution, which is defined as A solution; prepare 0.5L (10.3mM risedronic acid Acid + 55mM sodium hydroxide + 63 mM disodium hydrogen phosphate) solution, defined as B solution, A solution and B solution were filtered with a 0.22 μm filter membrane for later use.
[0147] (2) Preparation of risedronate zinc aluminum adjuvant FH002C suspension:
[0148] Prepare the risedronate zinc aluminum adjuvant by co-precipitating the A and B solutions according to the volume ratio of 1:1, that is, the prepared B solution is added drop by drop according to the volume ratio of 1:1 until A is completely added A suspension formed from a solution. The suspension obtained after mixing was sterilized once at 121°C for 60 minut...
preparation example 2
[0149] Preparation Example 2: Preparation of risedronate zinc aluminum adjuvant (FH002C) with different ratios of zinc, aluminum and risedronic acid
[0150] (1) Solution preparation:
[0151]With the molar concentration of Zn as 1, the designed Zn / Al / risedronic acid molar concentration ratio is 1: 0.02: 0.1, 1: 0.02: 0.15, 1: 0.02: 0.22, 1: 0.02: 0.33, 1: 0.02: 0.5, 1:0.02:1 and 1:0.05:0.1, 1:0.05:0.15, 1:0.05:0.22, 1:0.05:0.33, 1:0.05:0.5, 1:0.05:1 and 1:0.1:0.1, 1: 0.1: 0.15, 1: 0.1: 0.22, 1: 0.1: 0.33, 1: 0.1: 0.5, 1: 0.1: 1 and 1: 0.15: 0.1, 1: 0.15: 0.15, 1: 0.15: 0.22, 1: 0.15:0.33, 1:0.15:0.5, 1:0.15:1 and 1:0.25:0.1, 1:0.25:0.15, 1:0.25:0.22, 1:0.25:0.33, 1:0.25:0.5, 1:0.25: 1 and 1:0.5:0.1, 1:0.5:0.15, 1:0.5:0.22, 1:0.5:0.33, 1:0.5:0.5, 1:0.5:1, prepare 0.5L (47mM zinc chloride+0.94, 2.35, 4.7, 7.05, 11.75, 23.5mM aluminum chloride) solution, defined as A solution; prepare 0.5L (4.7, 7.05, 10.3, 15.51, 23.5, 47mM risedronic acid + 35-250mM sodium hydroxide + 63 mM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com