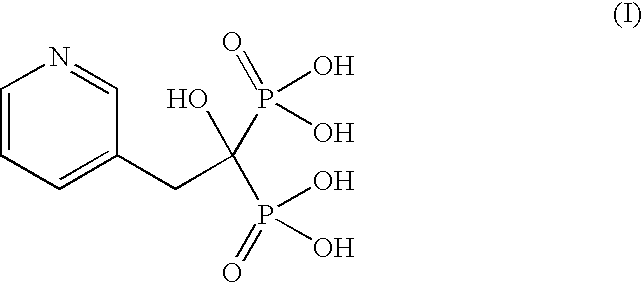
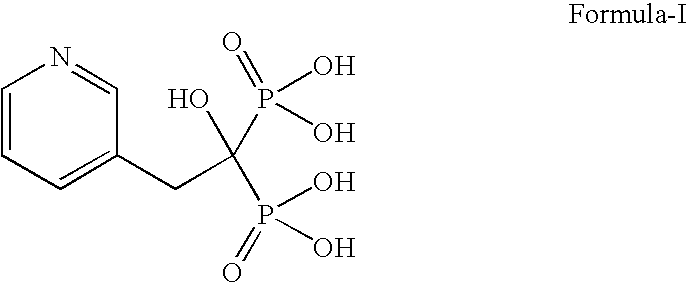
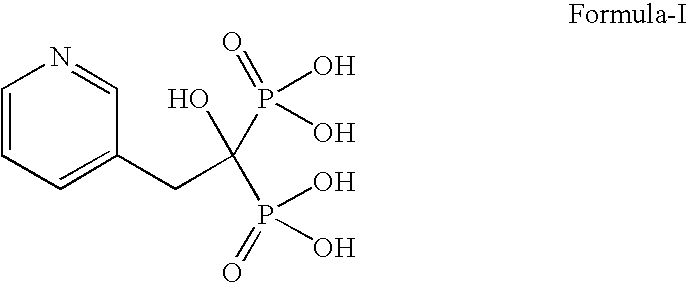
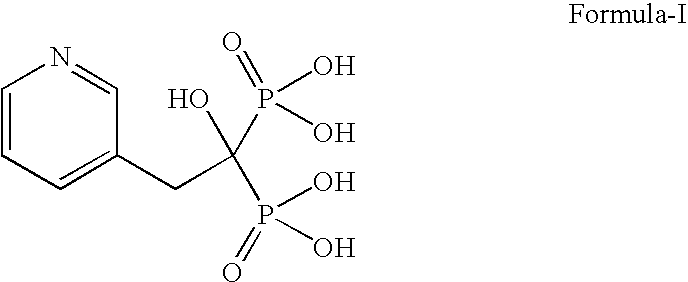
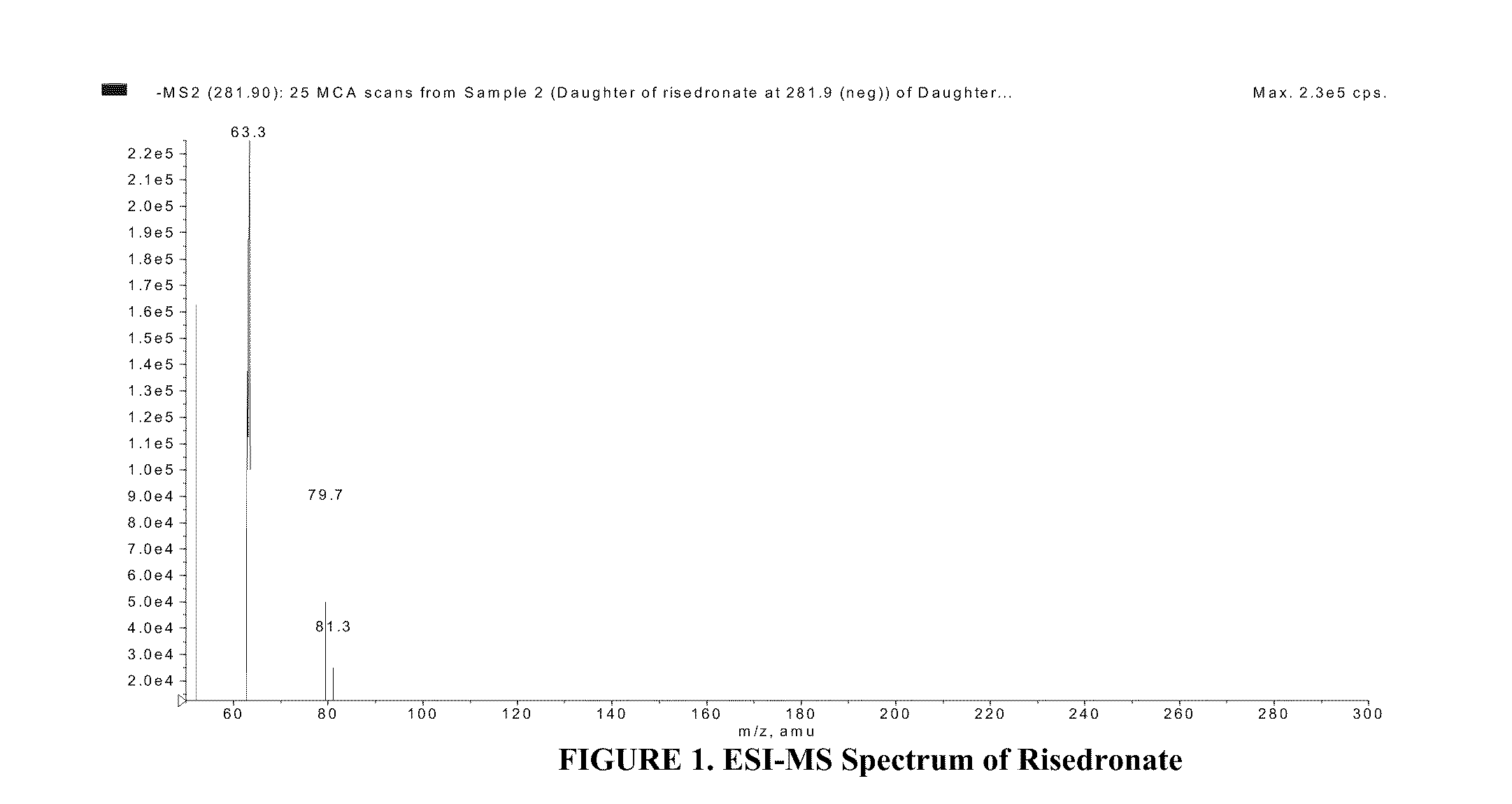
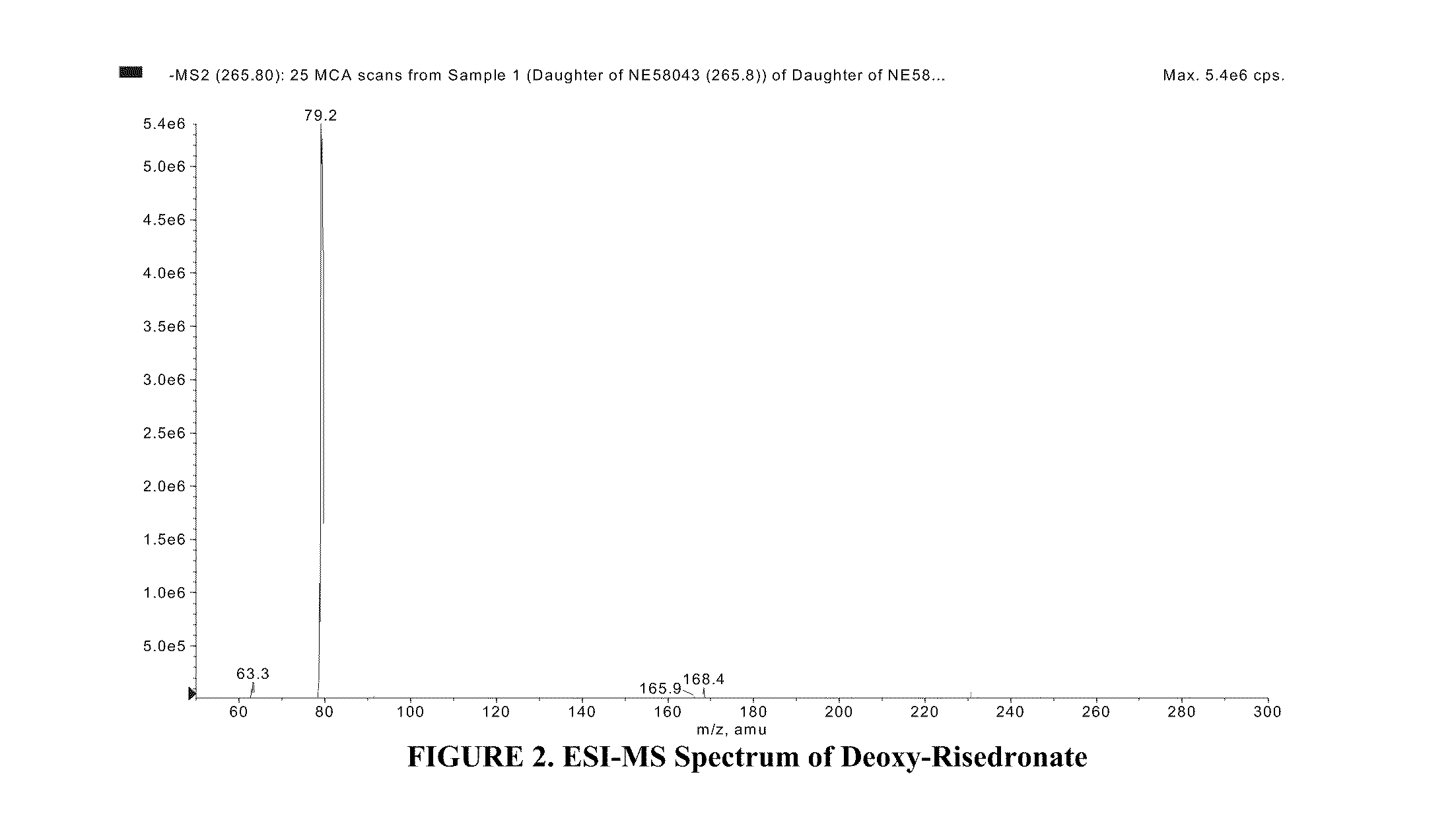
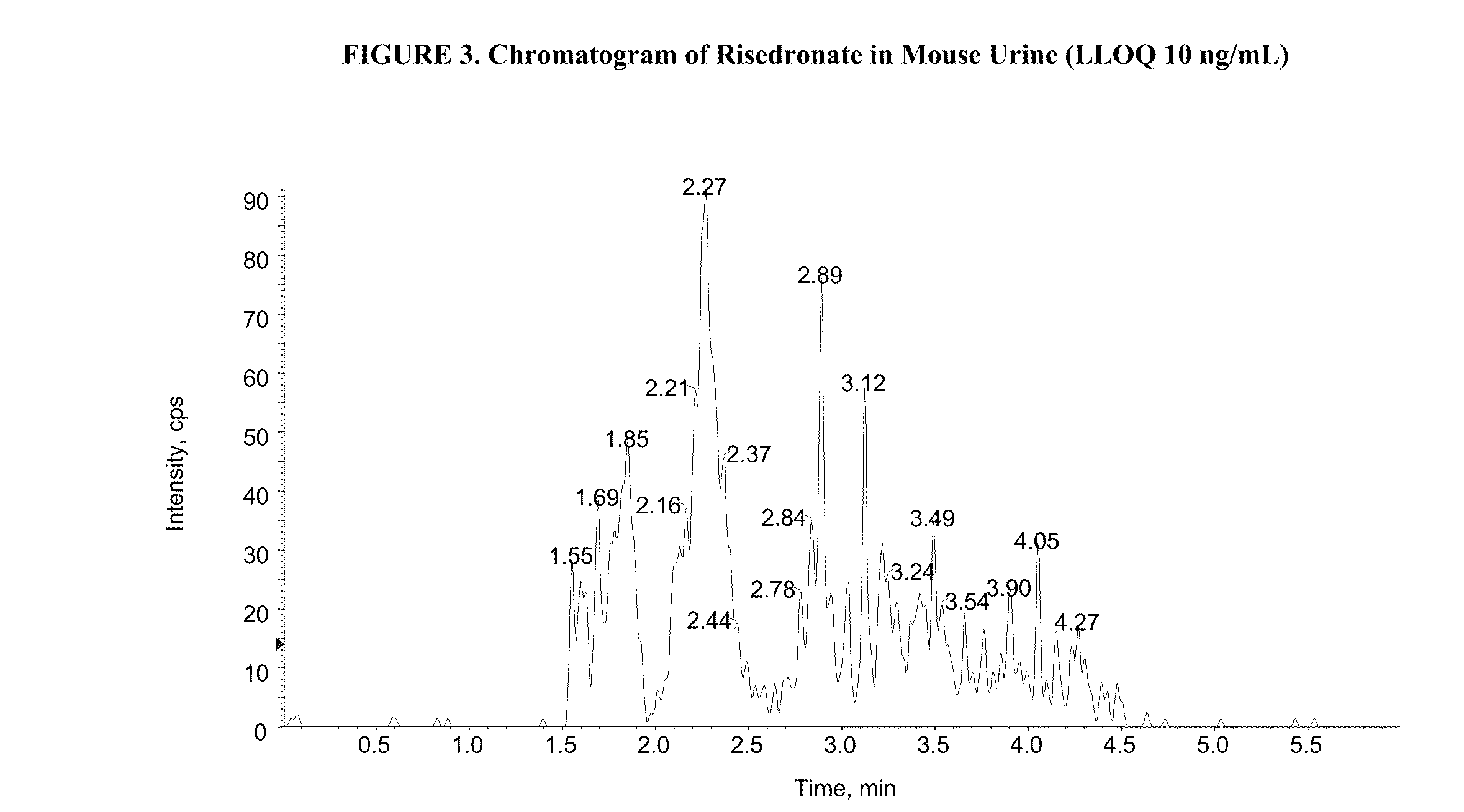
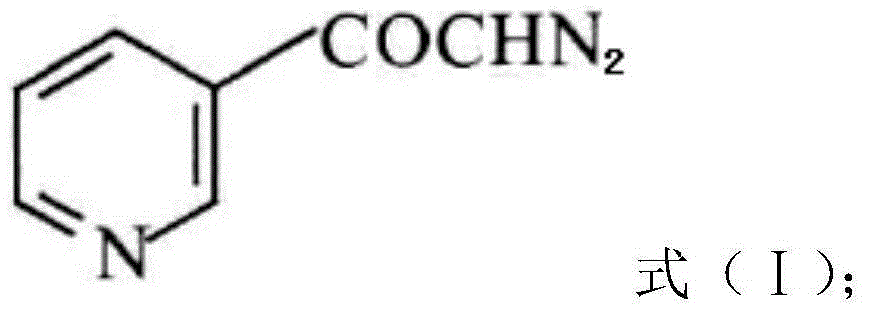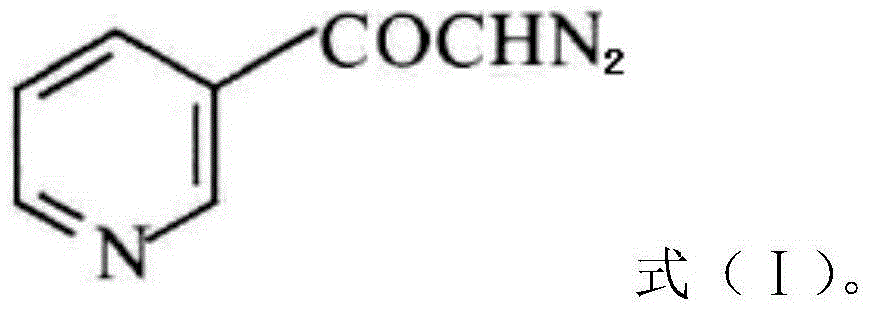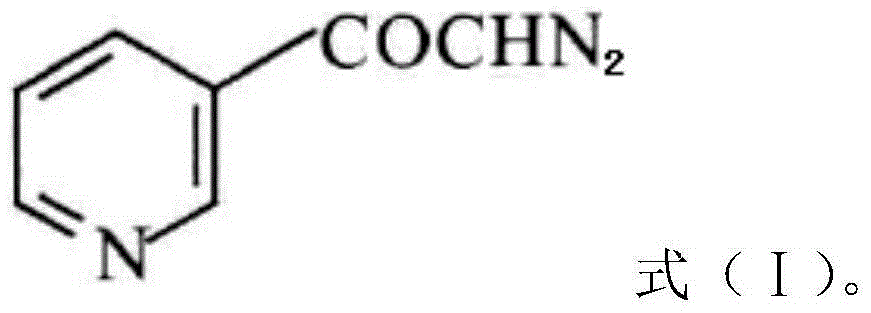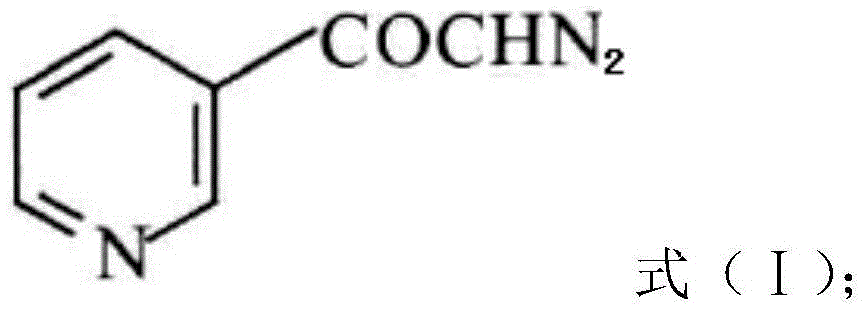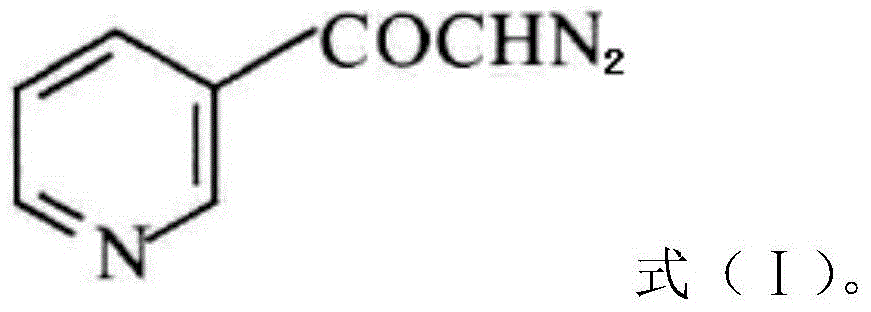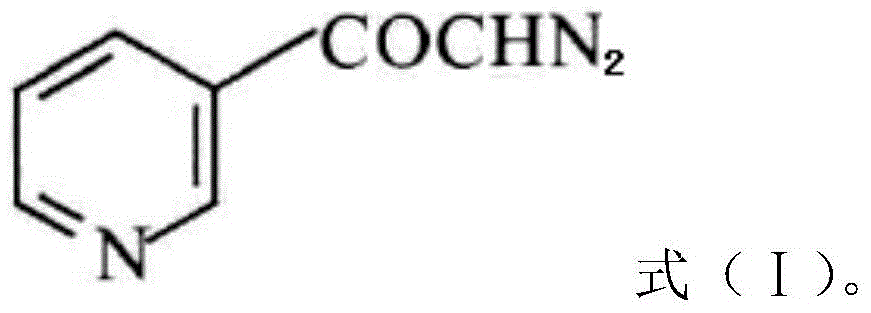Patents
Literature
30 results about "Risedronic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
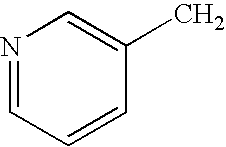
Risedronate is used by adults to treat a disease that weakens bones (Paget's disease).
Process for making bisphosphonic acids using diluents other than halogenated hydrocarbons
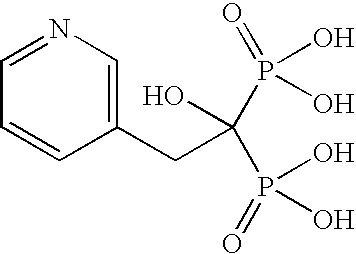
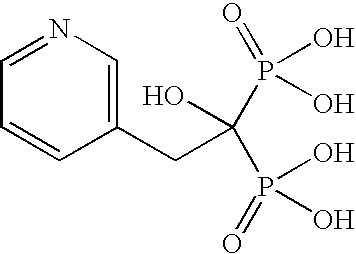
Provided is a novel method of making bisphosphonic acids, e.g. risedronic acid, including the step of combining a carboxylic acid, phoshorous acid, and a halophosphorous compound in the presence of a diluent that is an aromatic hydrocarbon or a silicone fluid. When the diluent is an aromatic hydrocarbon, a inert support or ortho-phosphoric acid codiluent is advantageously included.
Owner:TEVA PHARM USA INC
Novel Process For Making Bisphosphonic Acids Using Diluents Other Than Halogenated Hydrocarbons
Provided is a novel method of making bisphosphonic acids, e.g. risedronic acid, including the step of combining a carboxylic acid, phosphorous acid, and a halophosphorous compound in the presence of a diluent that is an aromatic hydrocarbon or a silicone fluid. When the diluent is an aromatic hydrocarbon, an inert support or ortho-phosphoric acid codiluent is advantageously included.
Owner:LIDOR HADAS RAMI +3
Method of determining risedronate in human plasma based on LC-MS/MS pre-derivatization and application thereof
InactiveCN110308217AThe sample pretreatment process is simpleShort runtimeComponent separationDerivatizationProtein C
The invention relates to a method of determining risedronate in human plasma based on LC-MS / MS pre-derivatization. The pre-derivatization comprises steps: 150 muL of a plasma sample is taken, 5.00 muLof an internal standard working solution is added, uniform mixing is carried out, 150 muL of 10% trichloroacetic acid is added, after 3 min of oscillation, centrifugation for 5 min at 13,000 rpm is carried out, 100 muL of a supernatant is taken, 57.0 muL of methanol-25% ammonia water (50 to 7, v / v) is added, and 3 min of oscillation is carried out; and 400 muL of trimethylsilyl diazomethane is added, oscillatory derivation for 4 h at 1600 rpm is carried out, and after standing, 2.00 muL of a lower solution is taken for sampling. In comparison with the prior art, a derivative reaction is directly carried out after protein precipitation, the risedronate sample preprocessing process is greatly simplified, and the method has the advantages of short running time, good selectivity and high sensitivity.
Owner:北京九昊医药科技有限公司
Novel process for making bisphosphonic acids using diluents other than halogenated hydrocarbons
Provided is a novel method of making bisphosphonic acids, e.g. risedronic acid, including the step of combining a carboxylic acid, phoshorous acid, and a halophosphorous compound in the presence of a diluent that is an aromatic hydrocarbon or a silicone fluid. When the diluent is an aromatic hydrocarbon, a inert support or ortho-phosphoric acid codiluent is advantageously included.
Owner:LIDOR HADAS RAMI +3
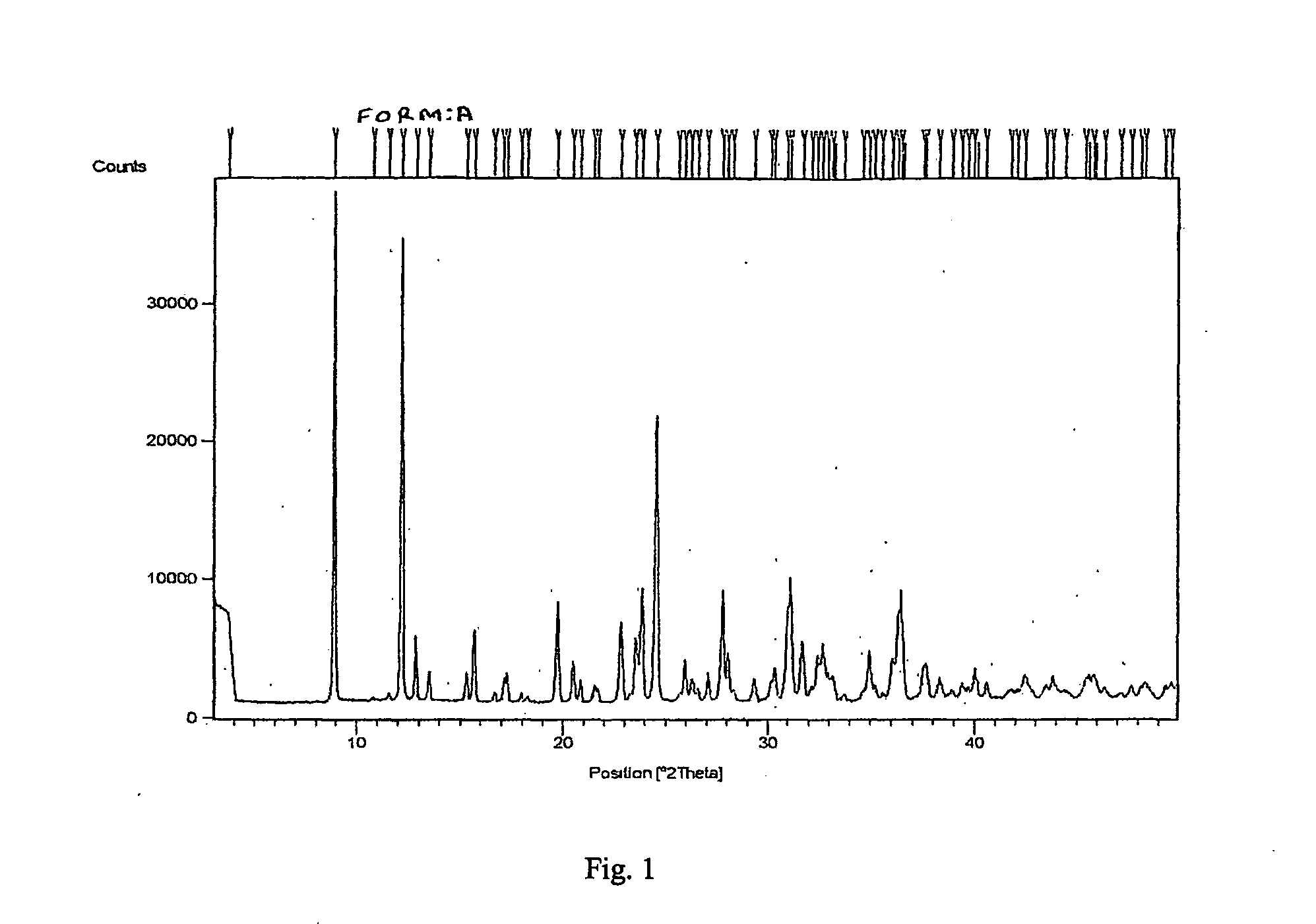
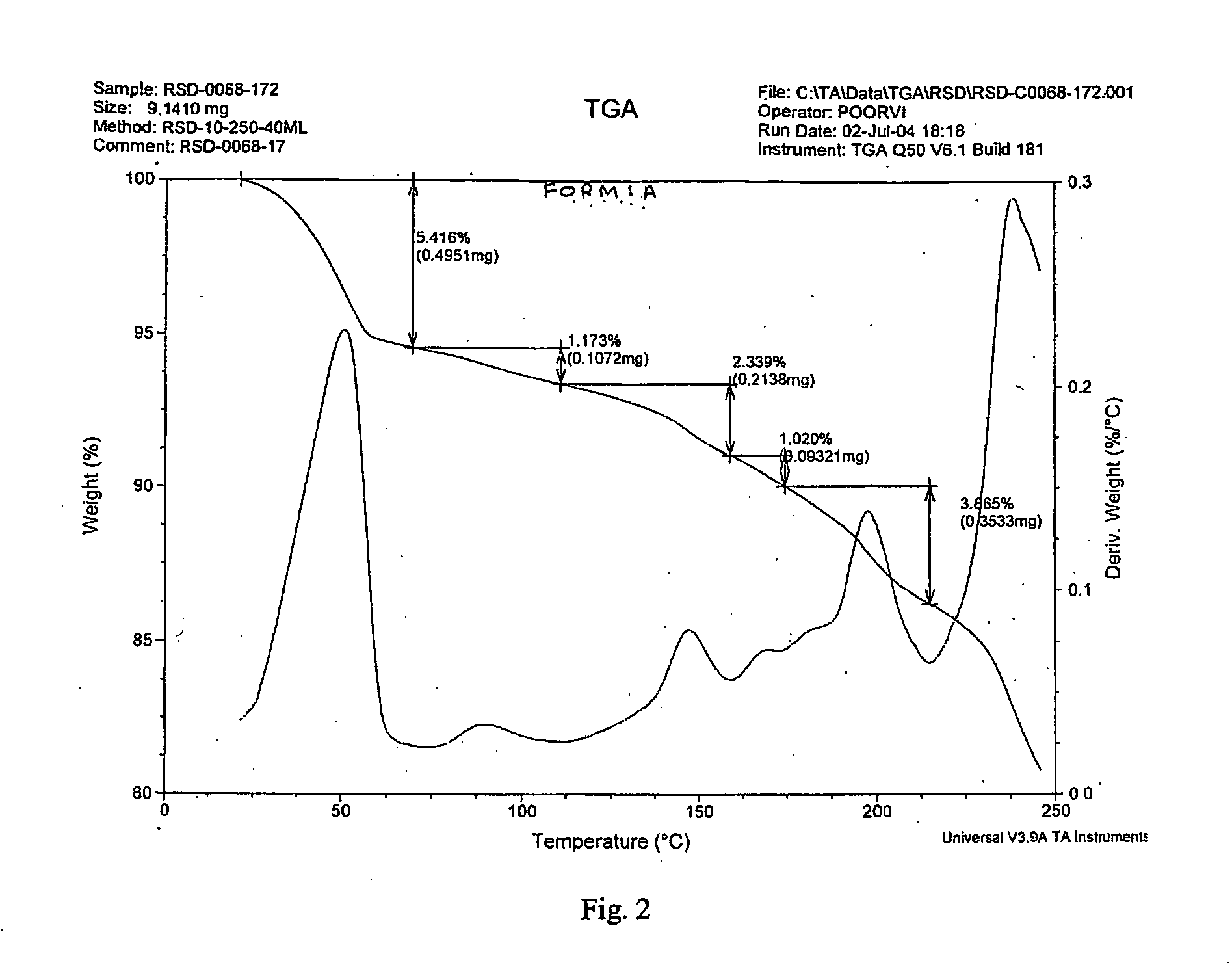
Process for Preparing a Pure Polymorphic Form of 3-Pyridyl-1-Hydroxyethylidine-1, 1-Bisphosphonic Acid Sodium Salt
InactiveUS20080300408A1High yieldSpeed up the processPhosphorus organic compoundsRisedronate SodiumRisedronic acid
This process in general relates to the novel process for preparing polymorphic forms of 3-pyridyl-1-hydroxyethylidine-1,1-bisphosphonic acid sodium salt (Risedronate Sodium) in particular risedronate Form A and B employing a solvent system in an appropriate ratio. An improved process for preparation of risedronic acid is also disclosed in the present invention.
Owner:JUBILANT ORGANOSYS LTD
Novel process
InactiveUS20090198062A1Economical and scalableHigh yieldPhosphorus organic compoundsOrganic solventBiochemistry
The present invention relates to a process for the preparation of bisphosphonic acids and salts thereof, in particular monosodium salts thereof. The invention also relates to the conversion of the bisphosphonic acids to their sodium salts using an aqueous-organic solvent system. The present invention further relates to the conversion of variable hydrate forms of risedronic acid monosodium salt into a pharmaceutically acceptable hemipentahydrate form by crystallization using an aqueous-organic solvent system.
Owner:GENERICS UK LTD
Process for the Preparation of Risedronate Sodium
InactiveUS20100317859A1High purityHigh yieldPhosphorus organic compoundsRisedronate SodiumRisedronic acid
Owner:ACTAVIS GRP PTC EHF
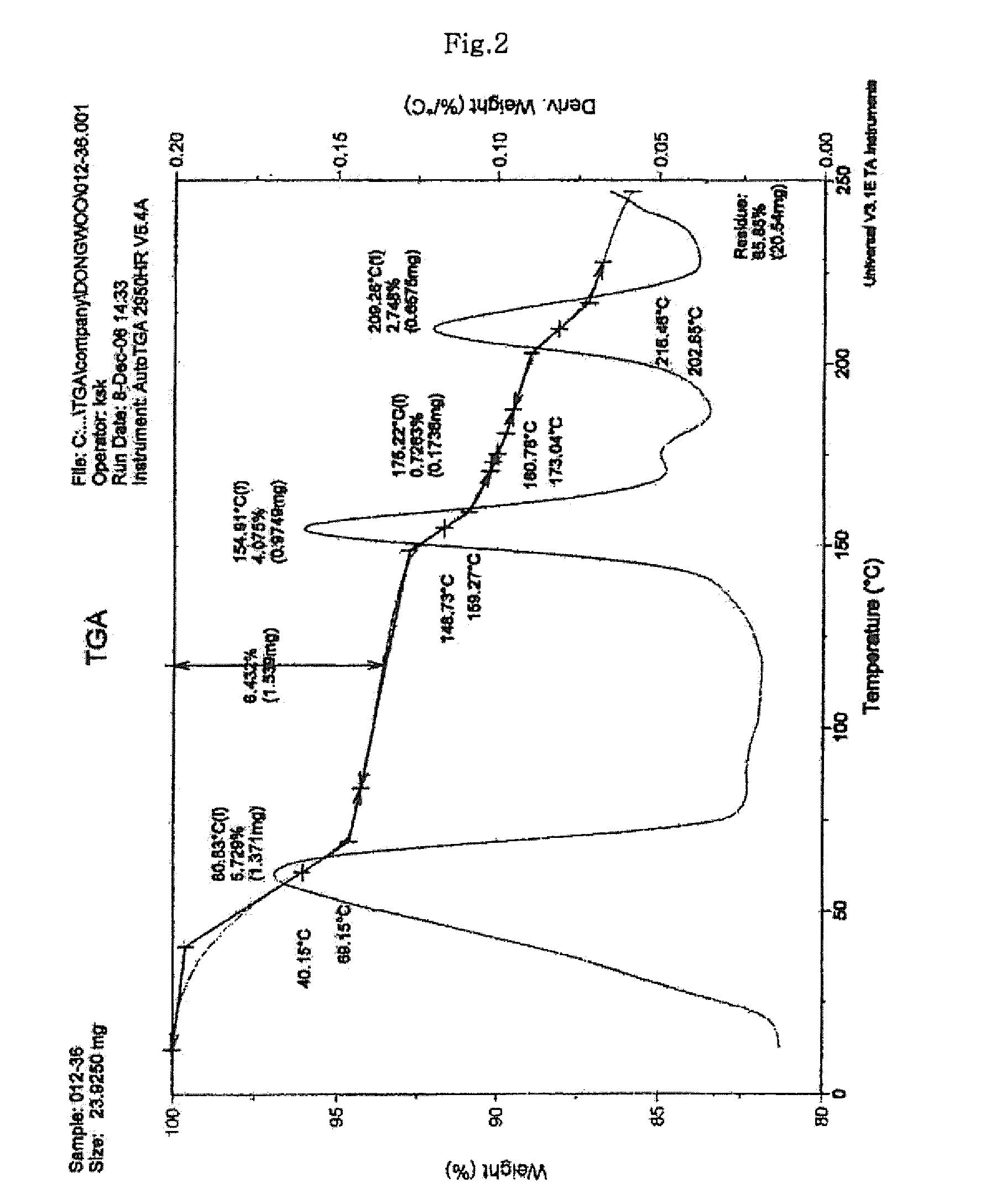
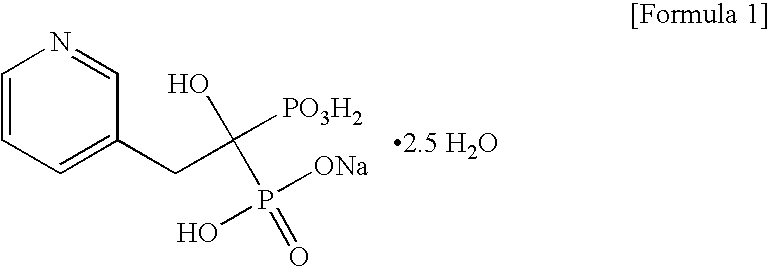
Process for preparing sodium risedronate hemipentahydrate
ActiveUS20100016592A1Skeletal disorderPhosphorus organic compoundsRisedronate SodiumAqueous solution
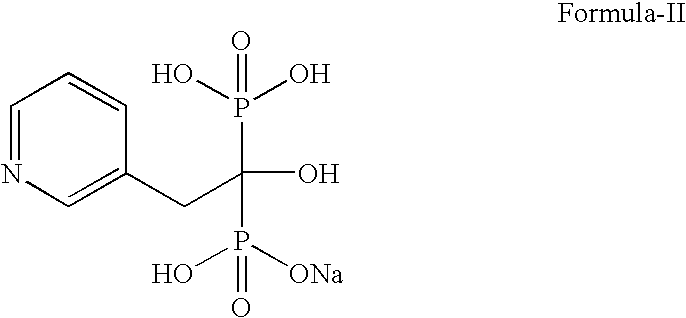
A novel process for preparing risedronate sodium hemipentahydrate represented by the following formula 1 using 2-(3-pyridyl)-1-hydroxyethane-1,1-bisphosphonic acid (risedronic acid) and an aqueous solution of risedronate sodium is disclosed.
Owner:DONGWOO SYNTECH CO LTD
Carboxyl contained NSAIDS (nonsteroidal anti-inflammatory drugs) salt
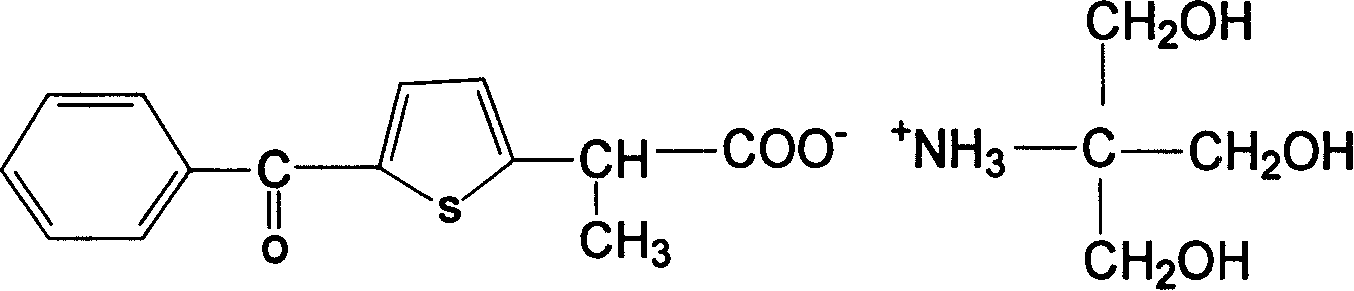
Disclosed are non-steroidal analgesic and analgesic anti-inflammatory agents containing carboxyl including sodium, calcium, zinc, magnesium, N-n-octylgucamine, Arginine, Lycine or Trometamol salts of Loxoprofen, Ketoprofen, Pranoprofen, Tiaprofenic acid, Butibufen, Omolofen, epoxy indene acid, Lobuprofen, Clofenamic acid, Clonixin, Fenoprofen, Benorilate, Flurbiprofen, Alminoprofen, Bucloxic acid, Sulindac, Zidometacin, Acemetacin, Ketorolac, Risedronic acid, Sulindac, Lonaprofen, aspirin, Florfenicol, tiaprofenic acid, overall evaluation shows that trometamol salts are the best choice in terms of physicochemical properties, solvability, stability, local irritation, blood vessel irritation, and bioavailability for oral administration.
Owner:陈文展
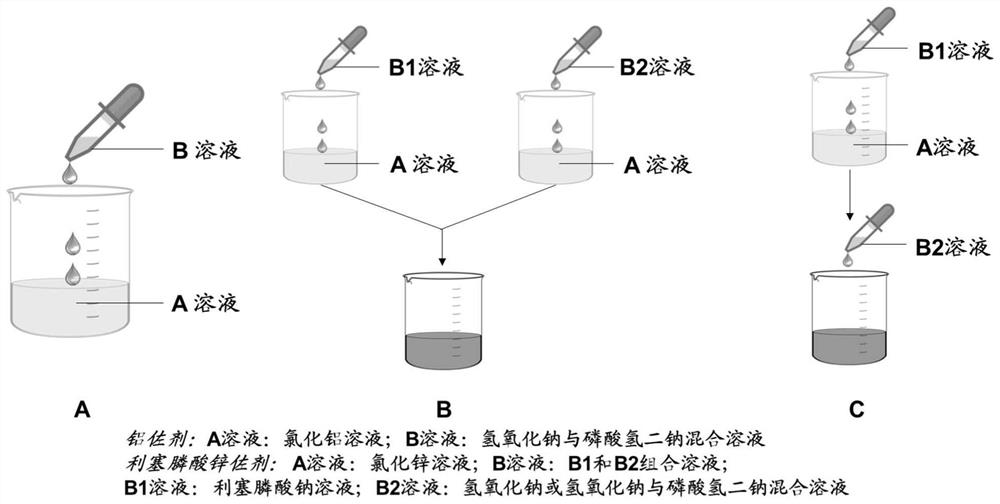
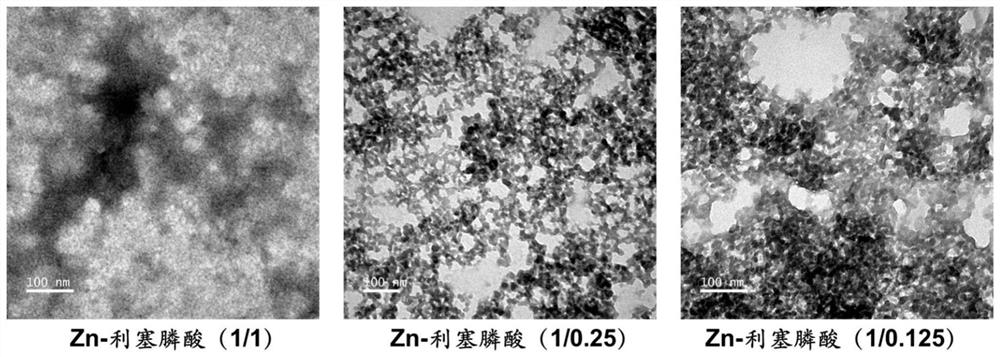
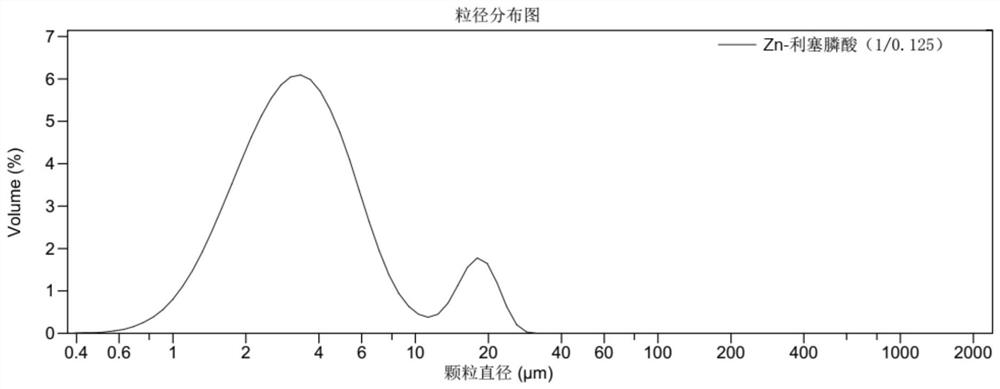
Preparation of risedronate zinc micro-nano adjuvant and application of adjuvant as vaccine adjuvant
The invention belongs to the technical field of medicines. Specifically, the invention relates to a risedronate zinc micro-nano adjuvant which is formed by mineralizing zinc ions and risedronate as main components and has a slow release function and application of the adjuvant in a vaccine adjuvant. The invention also relates to a preparation method of the risedronic acid micro-nano adjuvant. Theinvention also relates to a chemical composition, the vaccine adjuvant and a vaccine composition containing the risedronic acid micro-nano adjuvant. The invention also relates to application of the risedronic acid micro-nano adjuvant as the vaccine adjuvant.
Owner:XIAMEN UNIV +1
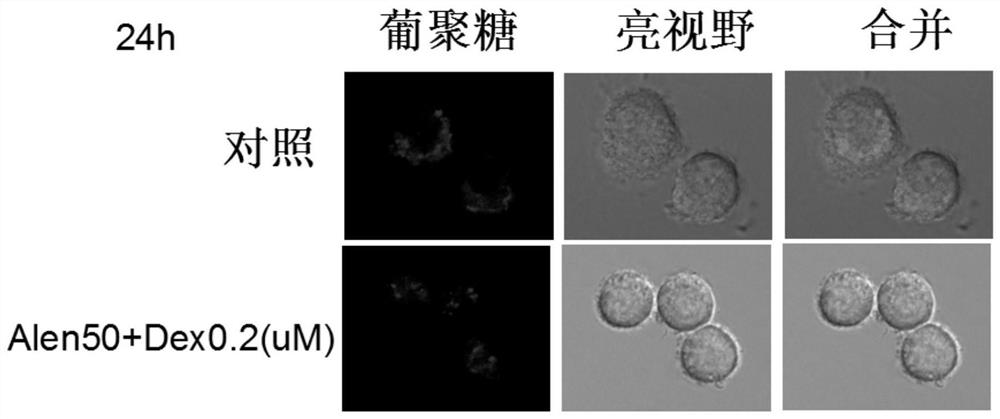
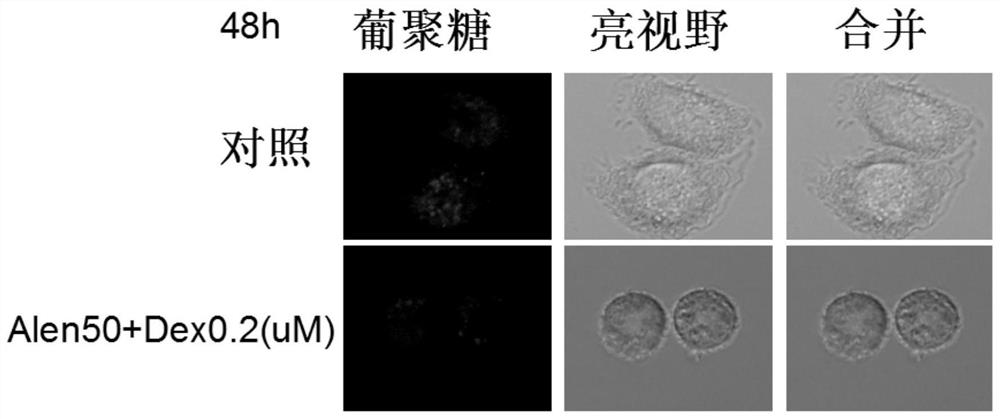
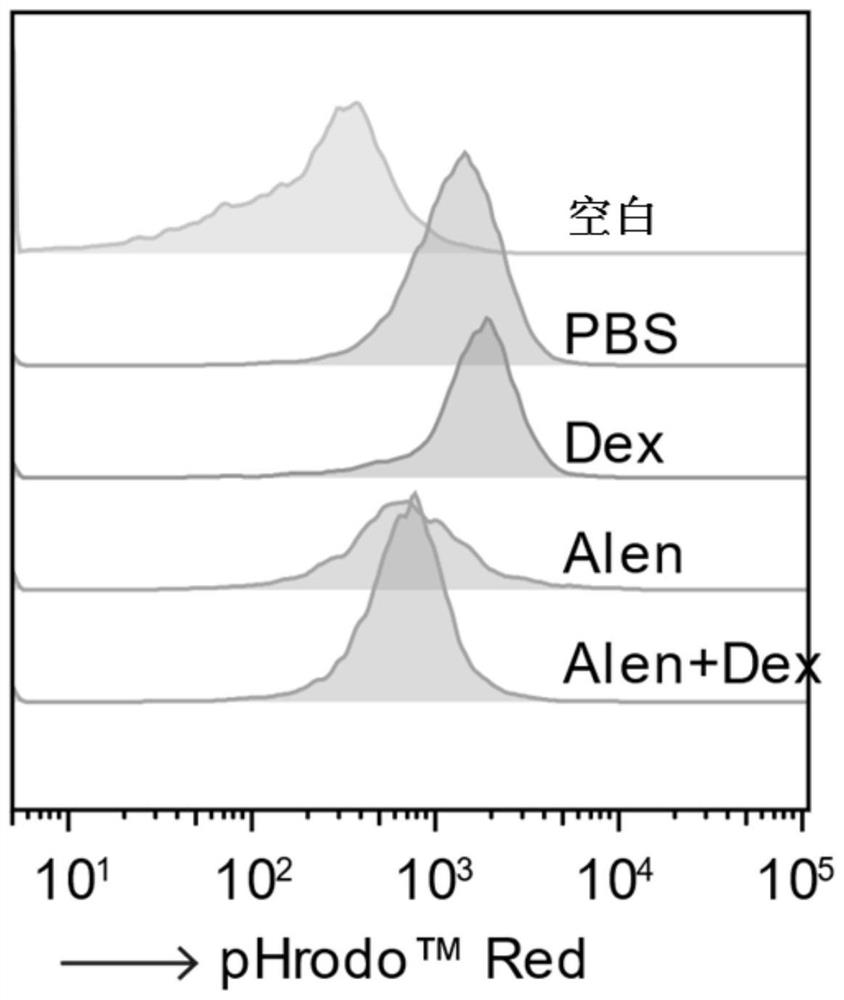
Application of nitrogenous bisphosphonate combined glucocorticoid in prevention or treatment of viral pneumonia
ActiveCN114028571AOrganic active ingredientsPharmaceutical delivery mechanismPropanoic acidFluticasone propionate
The invention provides application of nitrogenous bisphosphonate combined glucocorticoid in prevention or treatment of viral pneumonia. In particular, the nitrogenous bisphosphonate combined glucocorticoid is used to treat or ameliorate coronavirus infections, wherein the nitrogen-containing bisphosphonate is selected from pamironate, alendronate, risedronate, ibandronate, zoledronate, minodronate and pnaphonate, and the glucocorticoid is selected from the group consisting of dexamethasone, rimesolone, prednisolone, fluorometholone, hydrocortisone, mometasone, fluticasone, beclomethasone, flunivoxone, antiphlogistic, fluticasone propionate, beclomethasone dipropionate, budesonide and mometasone furoate.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
Novel Process For Preparing Risedronic Acid
The present invention relates to a process for preparing risedronic acid comprising the step of combining a 3-pyridyl acetic acid or a salt thereof, phosphorous acid, and a halophosporous compound selected from PCl3, PClS, POCl3, PBr3, POBr3, and PBr5 in the presence of a diluent that is either a bicyclic aliphatic hydrocarbon or a substituted cyclic aliphatic hydrocarbon or a mixture thereof, in combination with a codiluent, that is orthophosphoric acid
Owner:ALKEM LAB LTD
Preparation method of risedronate sodium
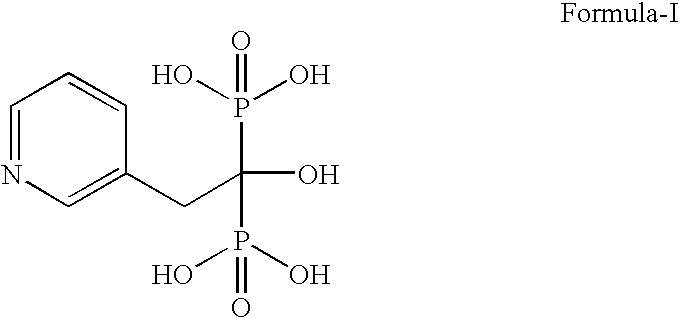
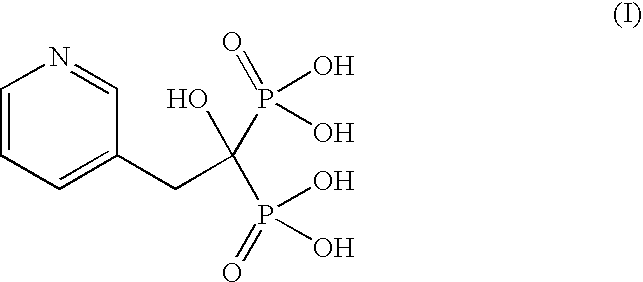
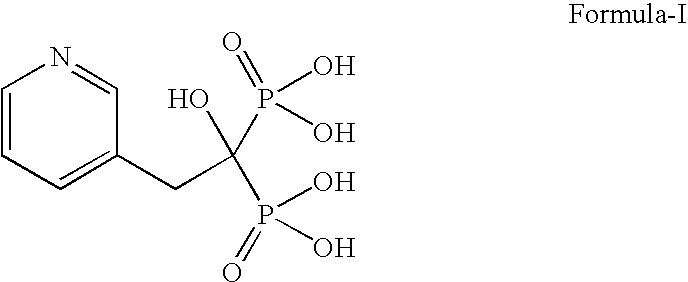
InactiveCN104628770AEasy to dissociateImprove responseGroup 5/15 element organic compoundsPhosphorous acidPhosphorus trichloride
The invention discloses a preparation method of risedronate sodium. The preparation method comprises the following steps of: (1) dissociating 3-pyridinecarboxylicacid chloride in 3-pyridinecarboxylicacid chloride hydrochloride; (2) reacting 3-pyridinecarboxylicacid chloride with diazomethane to generate a diazoketone compound represented by a formula (I); (3) generating 3-pyridylacetic acid under the common action of a catalyst and water; (4) adding hydrochloric acid, separating an organic phase from an inorganic phase, and evaporating and concentrating the inorganic phase to obtain 3-pyridylacetic acid hydrochloride; (5) converting 3-pyridylacetic acid hydrochloride into risedronic acid in the presence of phosphorus trichloride and phosphorous acid; and (6) reacting risedronic acid with sodium hydroxide solution to generate risedronate sodium. The preparation method of risedronate sodium disclosed by the invention has the advantages of being few in reaction step, short in reaction time, simple in post-treatment and higher in product yield.
Owner:LUOHE MEDICAL COLLEGE
Process for the preparation of pure risedronic acid or salts
InactiveUS20090182147A1Avoiding decantationAvoid isolationPhosphorus organic compoundsBiochemistryRisedronic acid
Owner:MS IND SWIFT LAB
Application of nitrogen-containing bisphosphonate in prevention or treatment of viral pneumonia
The invention provides application of nitrogen-containing bisphosphonates in prevention or treatment of viral pneumonia. In particular, the present regimen relates to new uses of nitrogen-containing bisphosphonates in the treatment or amelioration of coronavirus infection; wherein the nitrogen-containing bisphosphonate is selected from pamironate, alendronate, risedronate, ibandronate, zoledronate, minodronate and pnaphonate. The invention further discloses a preparation method of the nitrogen-containing bisphosphonate.
Owner:THE INST OF BASIC MEDICAL SCI OF CHINESE ACAD OF MEDICAL SCI
Risedronic acid synthesized by one-pot process
The invention provides a risedronic acid synthesized by a one-pot process. The risedronic acid is synthesized from 3-acetylpyridine as an initial material through Willgerodt-Kindle reaction, hydrolysis, diphosphonation and acidification employing the one-pot process. The risedronic acid has the advantages of being simple and convenient to operate, few in three wastes, mild and complete in reaction conditions, and suitable for industrial production, and has relatively high practical value; the reaction is finished in one container; the obtained product is stable in quality; and the purity can reach over 98%.
Owner:TOPFOND PHARMA CO LTD
Process for the preparation of pure risedronic acid or salts
InactiveUS8076483B2High purityHigh yieldBiocidePhosphorous compound active ingredientsBiochemistryRisedronic acid
Owner:MS IND SWIFT LAB
Quantitative determination of risedronate in urine by SPE-LC-MS-MS
The disclosure provides a method for quantitatively determining risedronate in a urine sample by adding an internal standard to the urine sample, applying the urine sample to a polymeric water-wettable reverse-phased sorbent preconditioned with methanol, washing the sorbent with TEA in water and formic acid in methanol, eluting risedronate with a mixture of methanol and water containing EDTA under vacuum, evaporating the eluted solution and reconstituting with a mixture of methanol and NH4OH buffer and analyzing the sample with a LC-MS / MS system.
Owner:SANOFI AVENTIS US LLC
Preventive agent or therapeutic agent for disease caused by abnormal bone metabolism
InactiveUS20100216747A1Good prevention effectGood treatment effectBiocidePhosphorous compound active ingredientsDiseaseTreatment effect
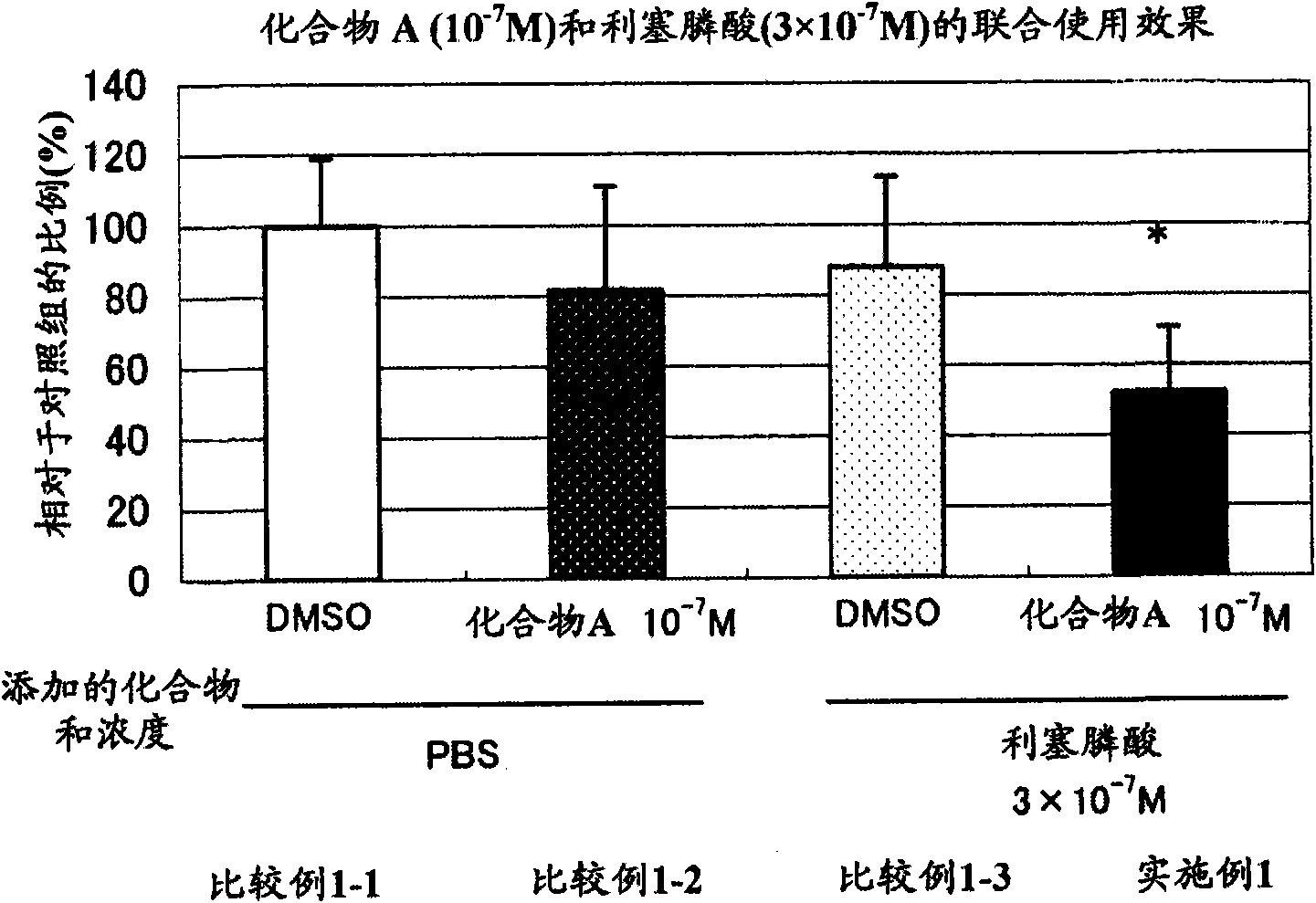
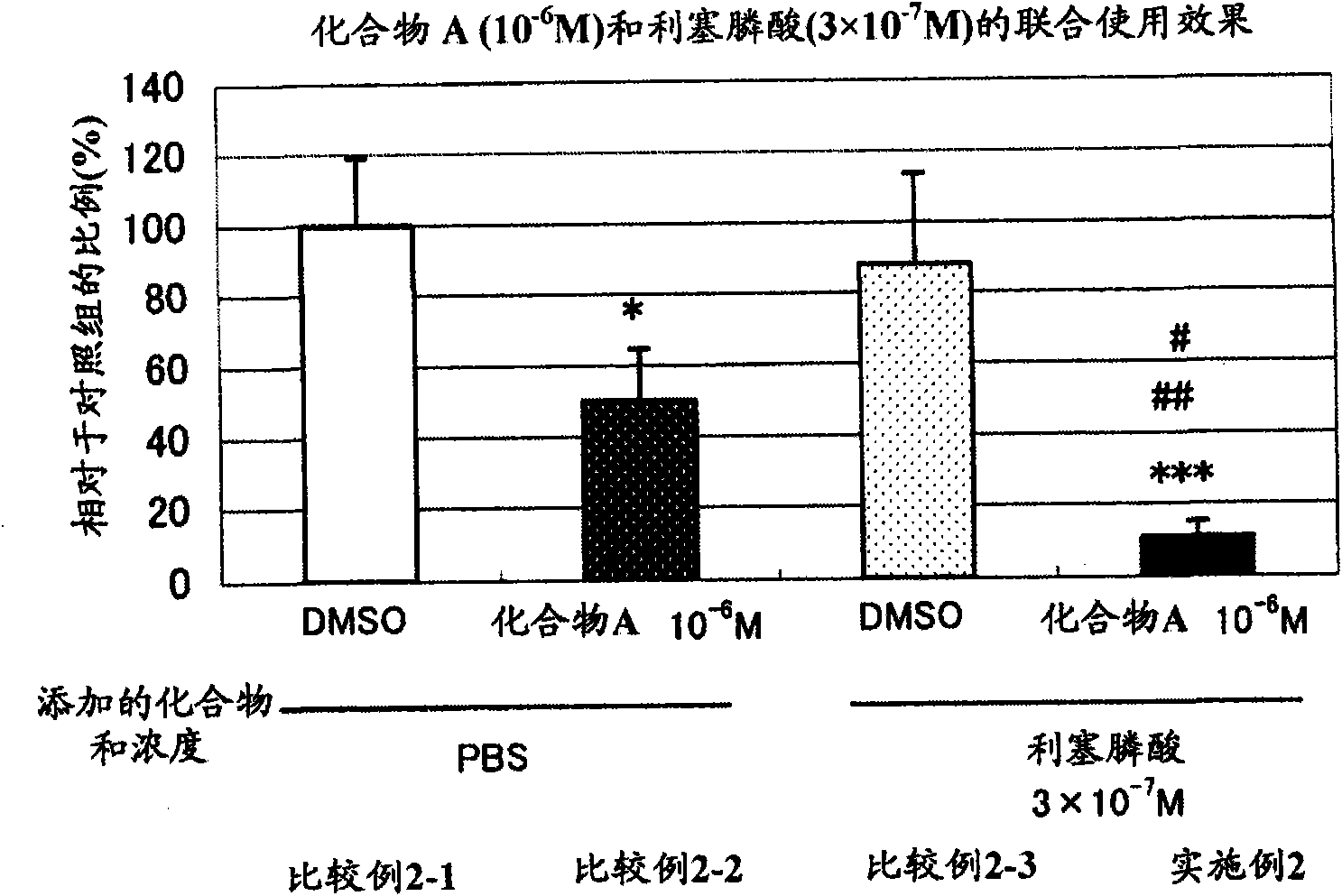
An object of the present invention is to provide a preventive drug and a therapeutic drug for diseases caused by abnormal bone metabolism, especially osteoporosis, which is more effective than conventional drugs. Combined use of N-hydroxy-4-{5-[4-(5-isopropyl-2-methyl-1,3-thiazol-4-yl)phenoxy]pentoxy}-benzamidine, or a salt thereof; and at least one compound selected from the group consisting of etidronic acid, clodronic acid, pamidronic acid, tiludronic acid, risedronic acid, minodronic acid, ibandronic acid, zoledronic acid, and salts thereof can exert higher bone resorption inhibitory effect and provide a preventive effect and a therapeutic effect for diseases caused by abnormal bone metabolism, especially osteoporosis as compared with administration of the respective agents respectively.
Owner:TEIJIN PHARMA CO LTD
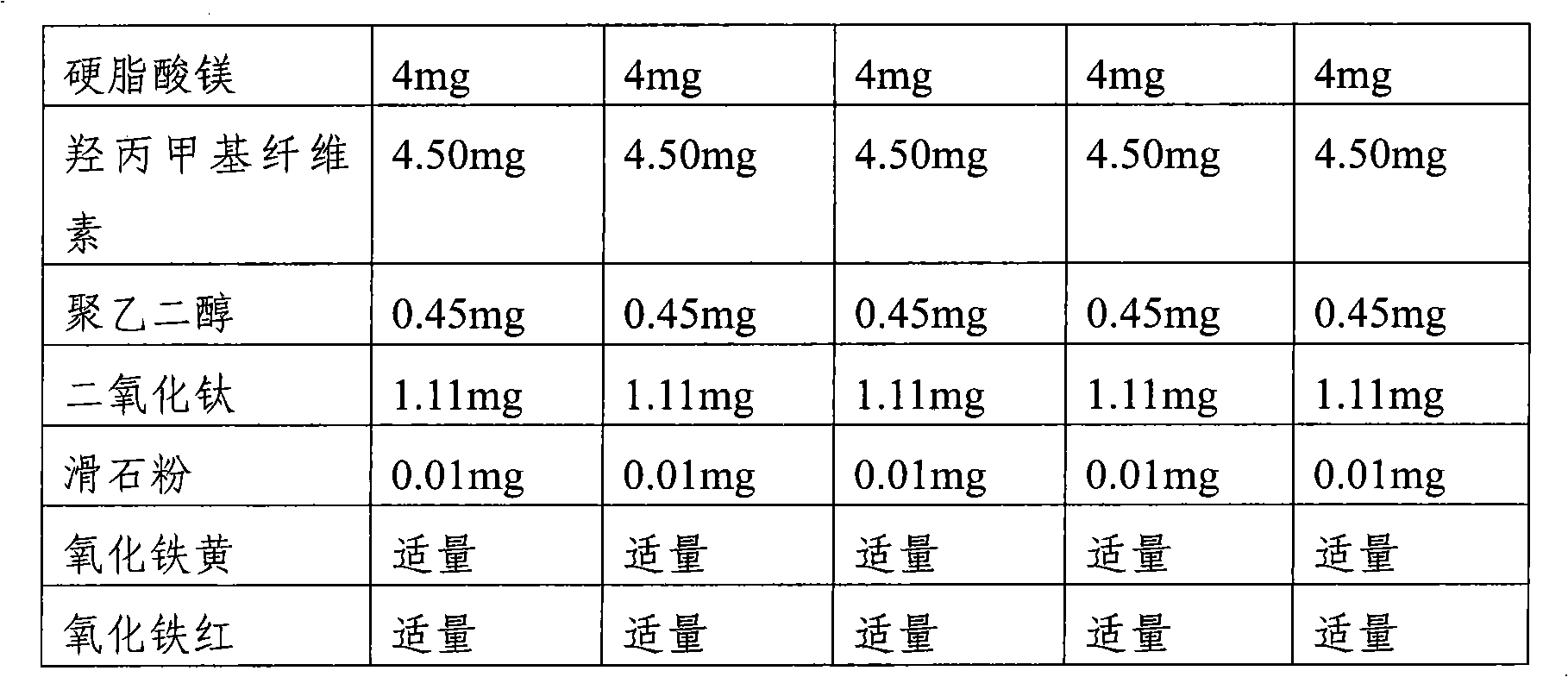
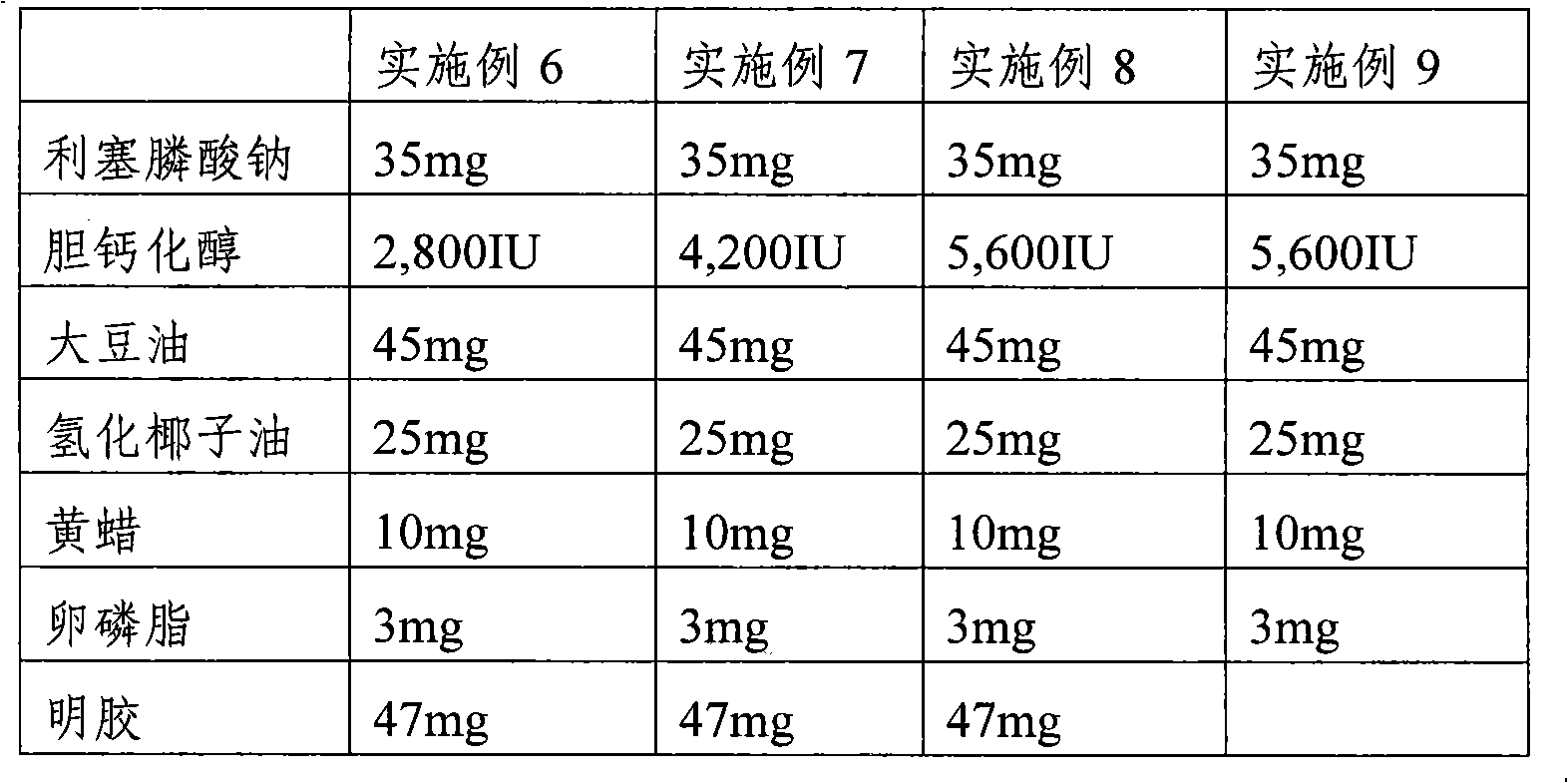
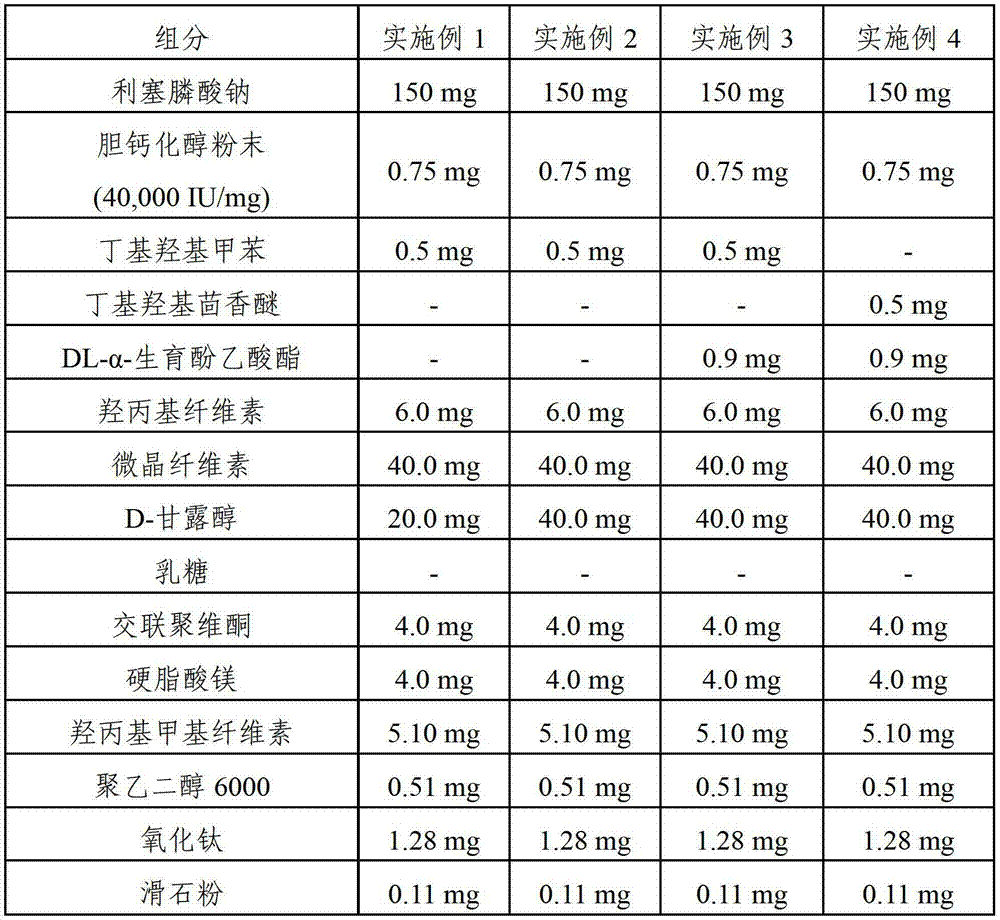
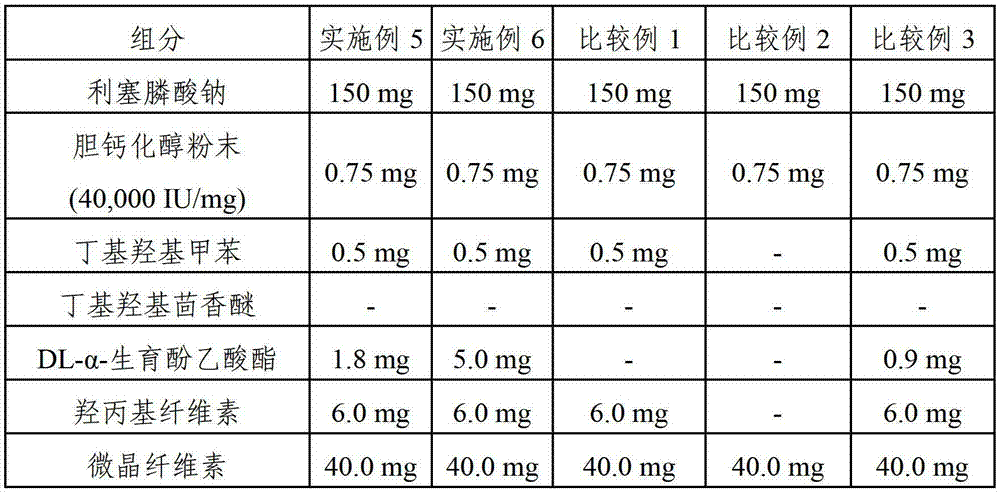
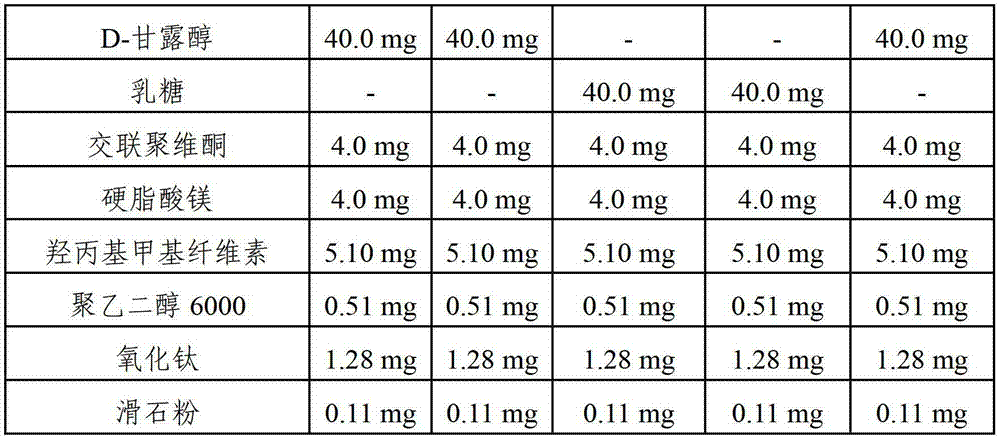
Pharmaceutical composition comprising risedronic acid or a salt thereof and vitamin D
InactiveCN102149387APromote absorptionGuaranteed uniformityOrganic active ingredientsSkeletal disorderHYDROGENATED COCONUT OILSolvent
The present invention provides a pharmaceutical composition for preventing or treating osteoporosis, comprising risedronic acid or a salt thereof and vitamin D. Further, the present invention provides an oral tablet obtained by tableting a mixture of risedronic acid or a salt thereof and granular vitamin D; and an oral capsule obtained by uniformly distributing risedronic acid or a salt thereof and vitamin D among the mixed solvent of soybean oil and hydrogenated coconut palm oil and filling a capsule with the resultant material.
Owner:HANLIM PHARMA CO LTD
Preventive agent or therapeutic agent for disease caused by abnormal bone metabolism
InactiveCN101808640AGood treatment effectOrganic active ingredientsInorganic phosphorous active ingredientsEtidronic acidTherapeutic effect
It is intended to provide a preventive agent or a therapeutic agent for a disease caused by abnormal bone metabolism, particularly for osteoporosis, which is more effective than ever before. By combined application of N-hydroxy-4-{5-[4-(5-isopropyl-2-methyl-1,3-thiazol-4-yl)-phenoxy]pentoxy}-benzamidine or a salt thereof and one or more compounds selected from the group consisting of etidronic acid, clodronic acid, pamidronic acid, tiludronic acid, risedronic acid, minodronic acid, ibandronic acid, zoledronic acid, and salts thereof, a higher bone resorption inhibitory action compared with the administration of a single compound is exhibited, and an excellent preventive effect and therapeutic effect for a disease caused by abnormal bone metabolism, particularly for osteoporosis can be obtained.
Owner:TEIJIN PHARMA CO LTD
One-pot Synthesis of Risedronic Acid
The invention provides a risedronic acid synthesized by a one-pot process. The risedronic acid is synthesized from 3-acetylpyridine as an initial material through Willgerodt-Kindle reaction, hydrolysis, diphosphonation and acidification employing the one-pot process. The risedronic acid has the advantages of being simple and convenient to operate, few in three wastes, mild and complete in reaction conditions, and suitable for industrial production, and has relatively high practical value; the reaction is finished in one container; the obtained product is stable in quality; and the purity can reach over 98%.
Owner:TOPFOND PHARMA CO LTD
Risedronate calcium complex and preparation method thereof
InactiveCN112724172AEasy to prepareReduce stressAntipyreticGroup 5/15 element organic compoundsCrystal systemPharmaceutical Substances
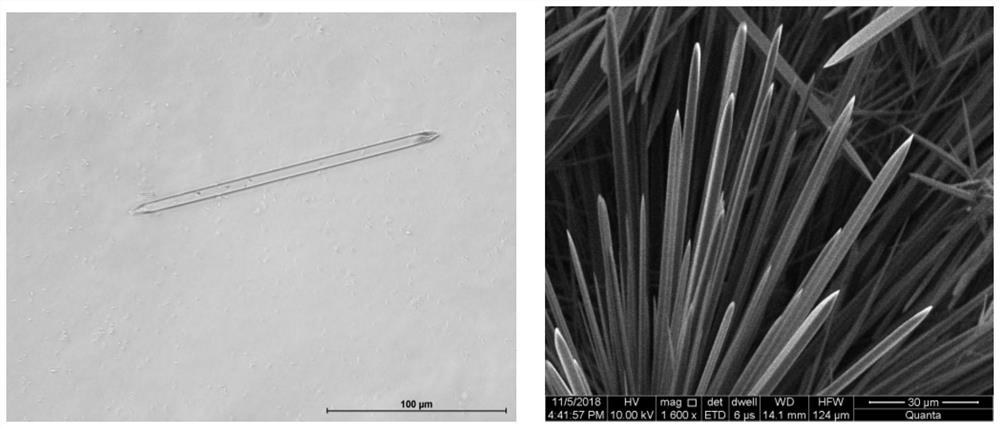
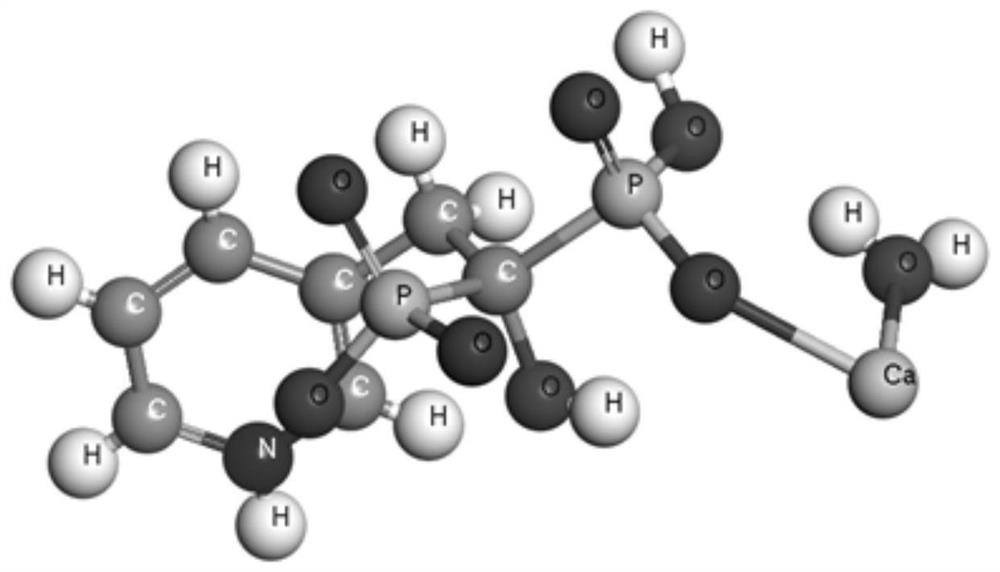
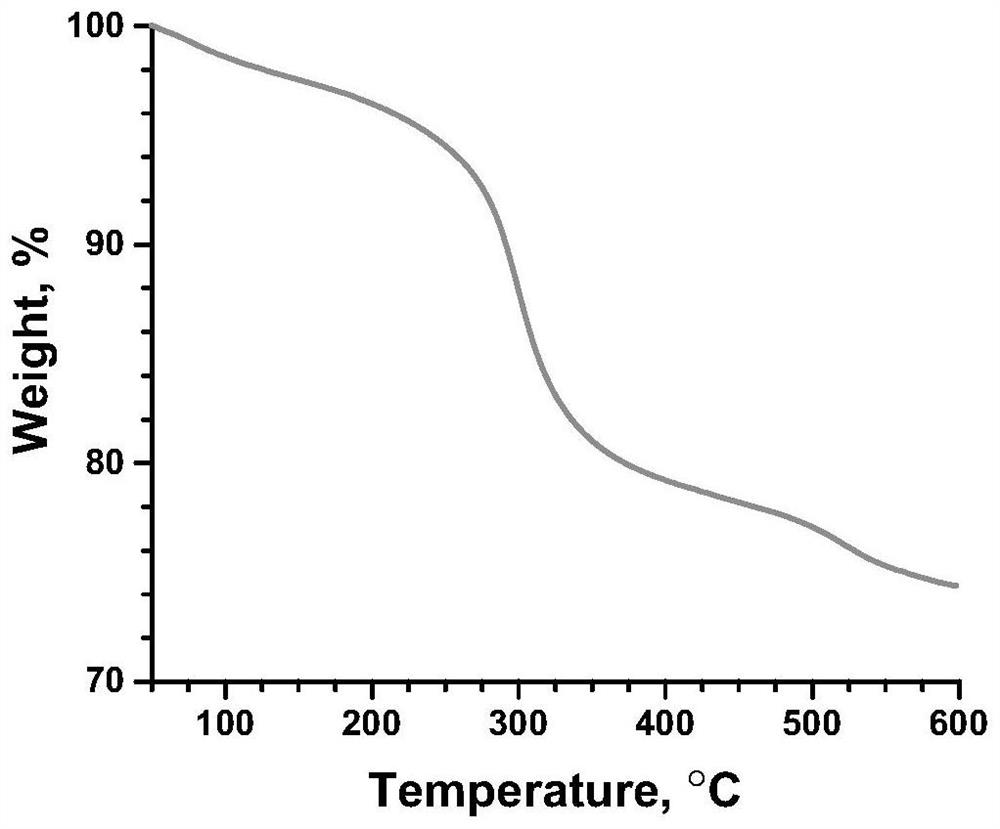
The invention discloses a risedronate calcium complex and a preparation method thereof, the chemical formula of the risedronate calcium complex is Ca (Ris) (H2O), Ca represents calcium, Ris represents risedronate, H2O represents water, the crystal system is an orthorhombic system, the space group is P212121, and the lattice constant z is 4. The preparation method comprises the following steps: by taking calcium salt and risedronate or risedronate sodium as raw materials and deionized water as a solvent, adjusting the pH value to 3-7 by using a sodium hydroxide solution, standing at 20-200 DEG C for 12-72 hours, filtering, washing and drying to obtain a strip-shaped transparent crystal, namely the risedronate calcium complex. The preparation method disclosed by the invention is extremely simple, moderate in crystal growth speed, small in stress, good in uniformity and very complete in appearance, and has important potential application prospects in the fields of drug sustained release, targeted delivery, bone tissue repair and the like.
Owner:SOUTH CHINA UNIV OF TECH
Adjuvant containing risedronate zinc aluminum and application thereof
PendingCN114504640AImproving immunogenicityGood effectSsRNA viruses negative-senseSsRNA viruses positive-senseAdjuvantMedicine
Owner:XIAMEN UNIV +1
A kind of preparation method of risedronate sodium
InactiveCN104628770BFacilitate dissociationImprove responseGroup 5/15 element organic compoundsPhosphorous acidRisedronate Sodium
The invention discloses a preparation method of risedronate sodium. The preparation method comprises the following steps of: (1) dissociating 3-pyridinecarboxylicacid chloride in 3-pyridinecarboxylicacid chloride hydrochloride; (2) reacting 3-pyridinecarboxylicacid chloride with diazomethane to generate a diazoketone compound represented by a formula (I); (3) generating 3-pyridylacetic acid under the common action of a catalyst and water; (4) adding hydrochloric acid, separating an organic phase from an inorganic phase, and evaporating and concentrating the inorganic phase to obtain 3-pyridylacetic acid hydrochloride; (5) converting 3-pyridylacetic acid hydrochloride into risedronic acid in the presence of phosphorus trichloride and phosphorous acid; and (6) reacting risedronic acid with sodium hydroxide solution to generate risedronate sodium. The preparation method of risedronate sodium disclosed by the invention has the advantages of being few in reaction step, short in reaction time, simple in post-treatment and higher in product yield.
Owner:LUOHE MEDICAL COLLEGE
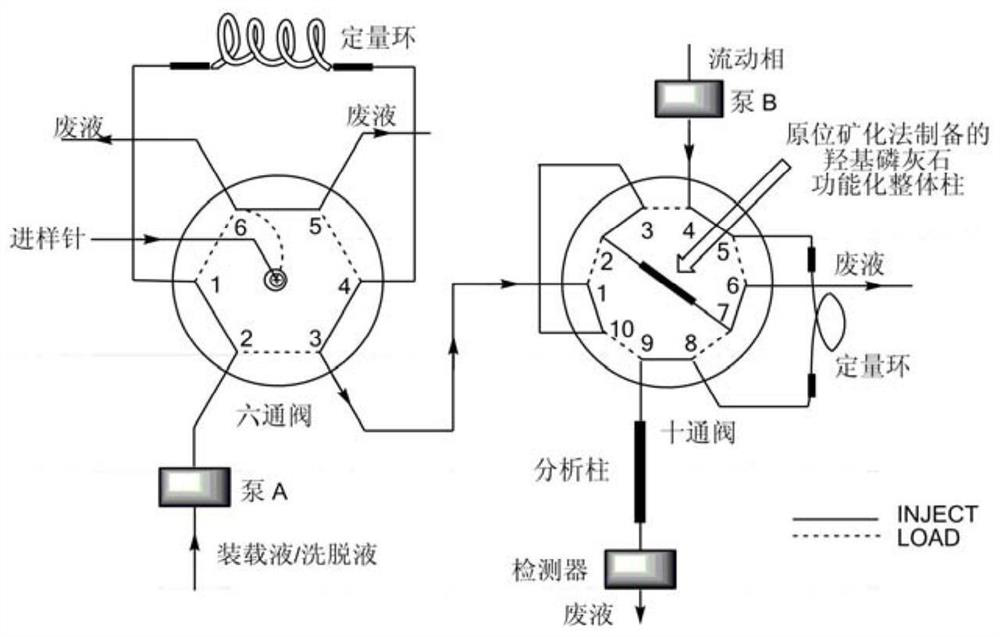
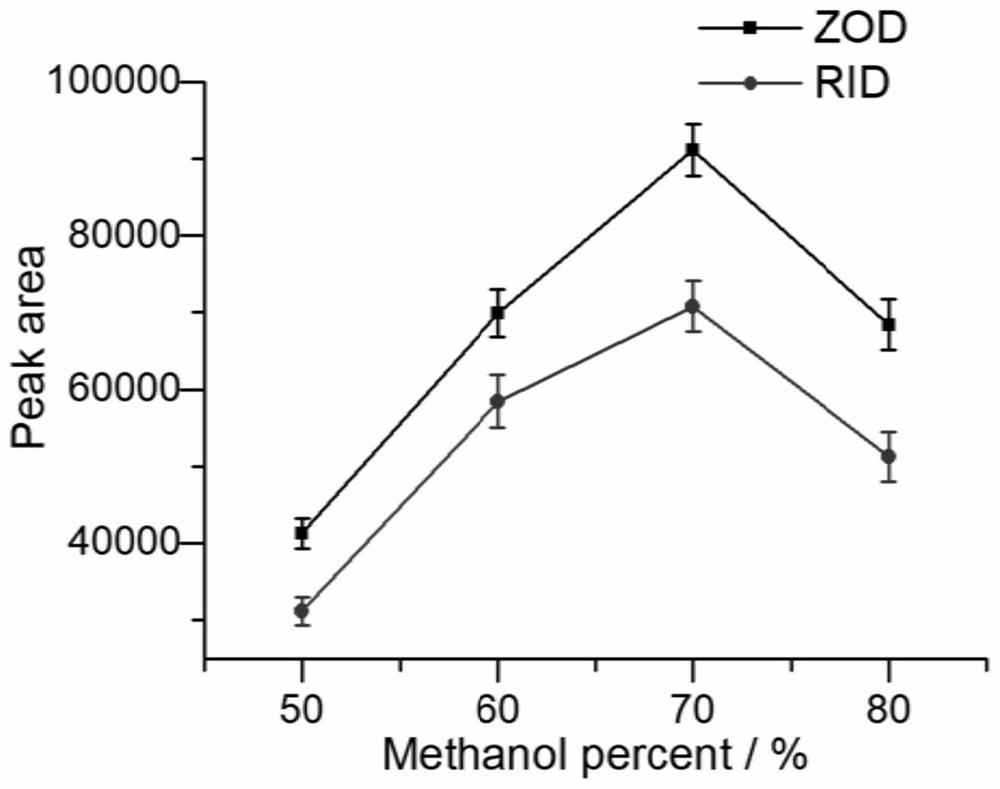
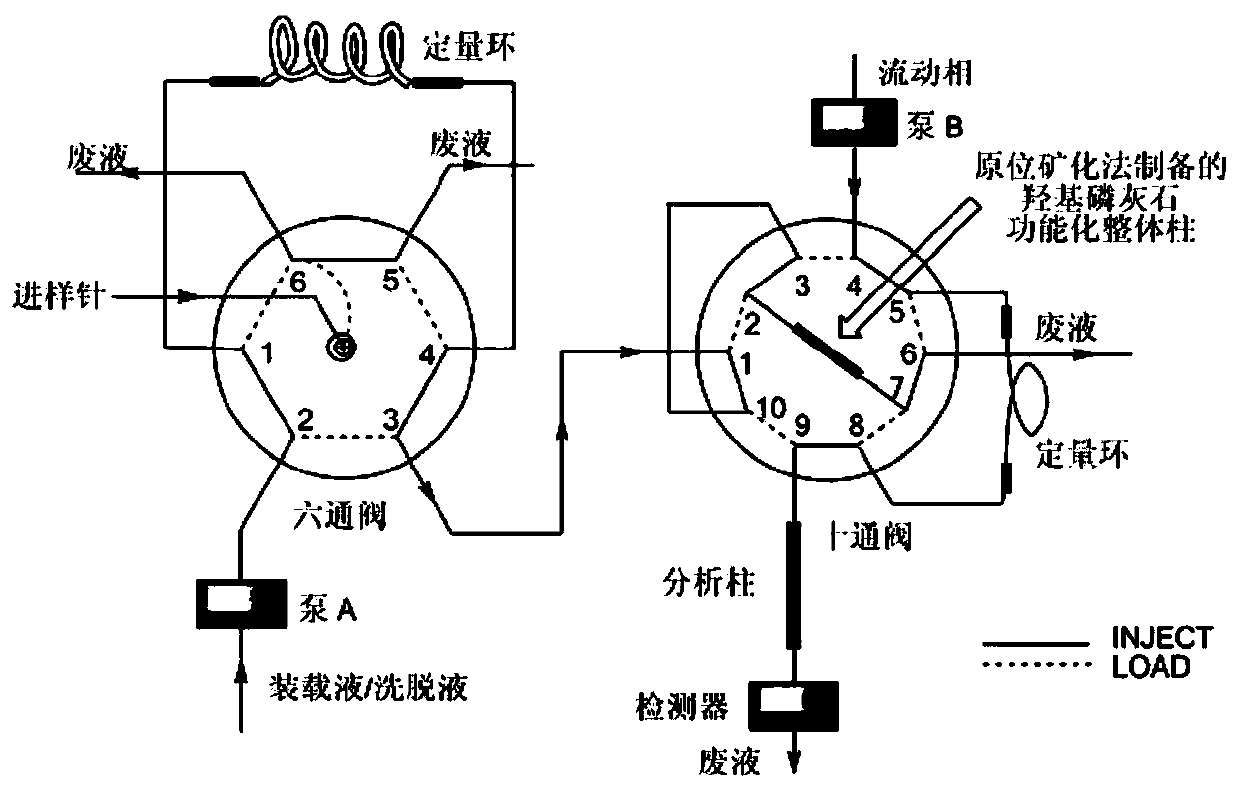
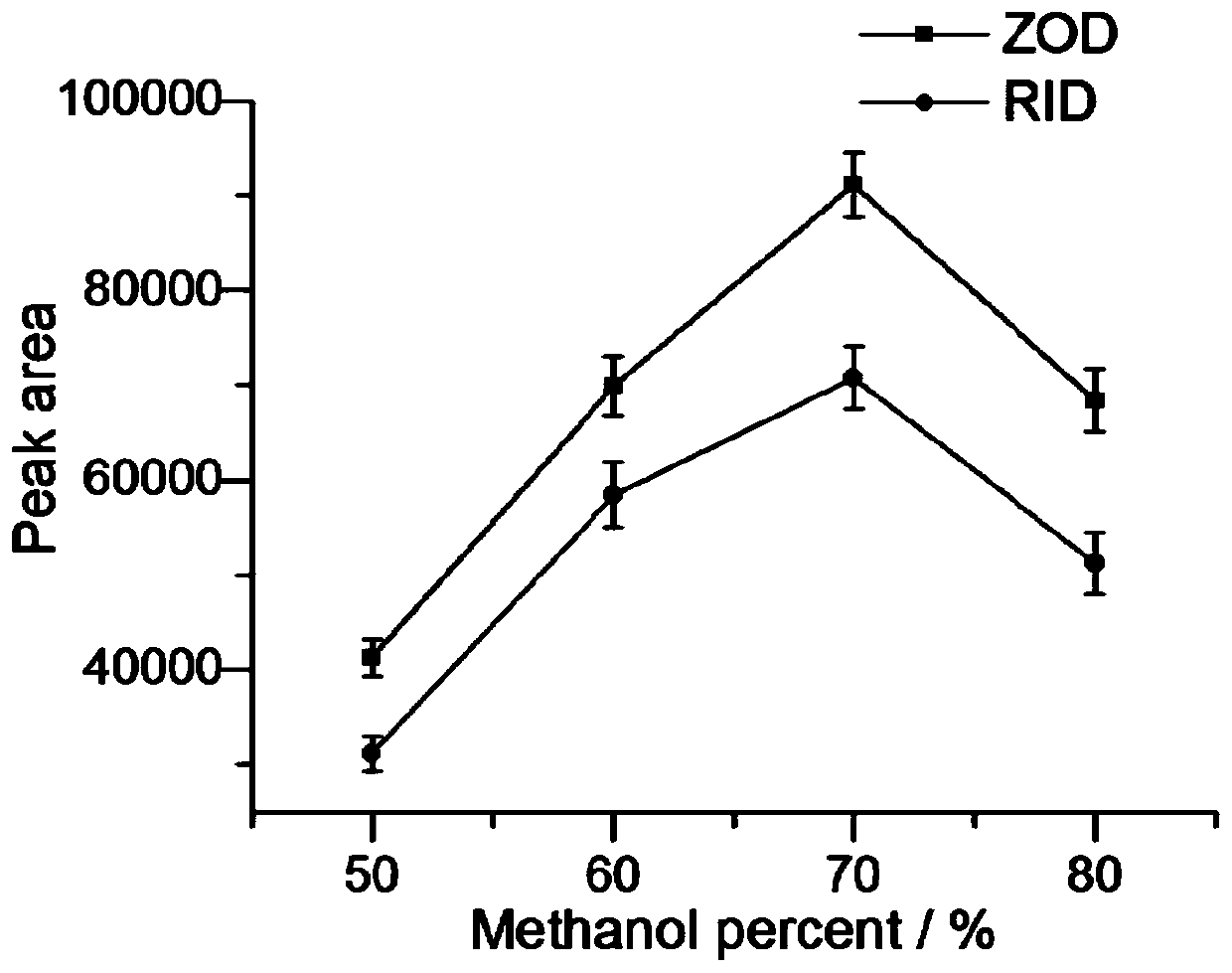
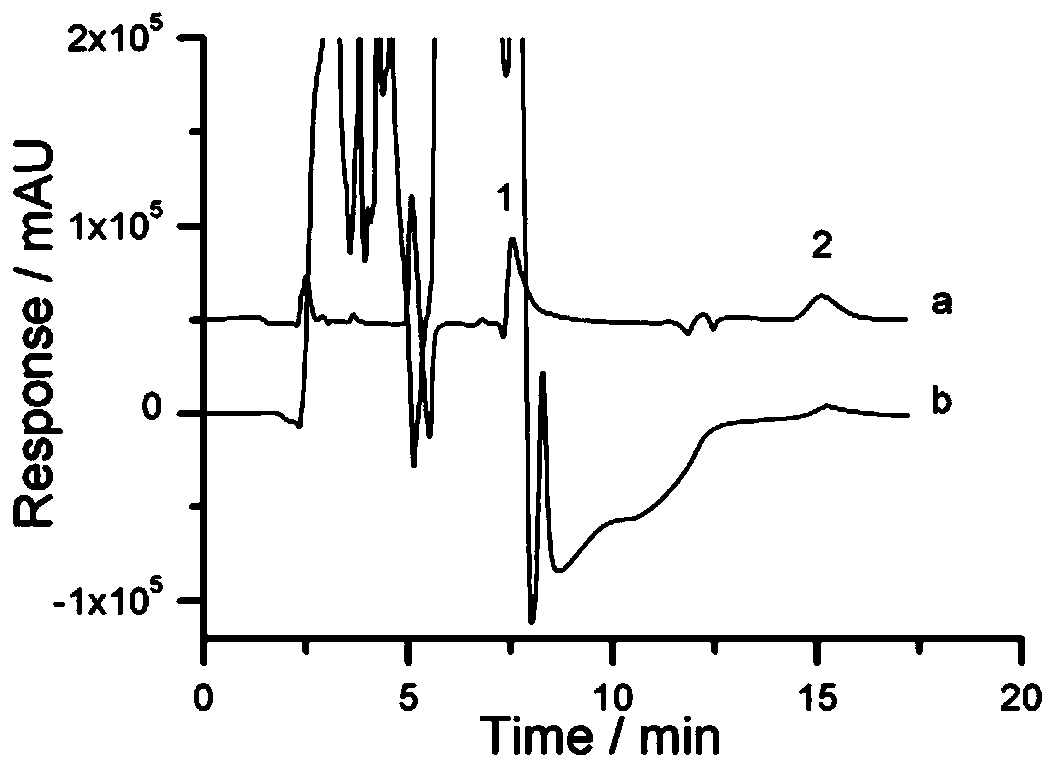
Solid-phase microextraction-high performance liquid chromatography online detection method for zoledronic acid and risedronic acid
ActiveCN110568114BImprove specific extraction abilityIncrease coverageComponent separationSolid sorbent liquid separationFluid phaseSolid-phase microextraction
The invention discloses a solid-phase microextraction-high performance liquid chromatography online combined detection method for zoledronic acid and risedronic acid. In the present invention, the hydroxyapatite functional monolithic column is firstly prepared by the in-situ mineralization method, and then the monolithic column is used as the solid phase microextraction monolithic column, combined with the solid phase microextraction-high performance liquid chromatography online system, to establish a Solid-phase microextraction-high performance liquid chromatography online detection method for zoledronic acid and risedronic acid. The invention utilizes the specific electrostatic interaction between the hydroxyapatite on the surface of the monolithic column and the P-C-P structure of the analysis object to realize the efficient extraction and enrichment of zoledronic acid and risedronic acid, and eliminate The interference of impurities in the sample matrix on the analytical detection. The method of the invention is simple, the technique is ingenious, the required instruments are highly popular, and it is easy to popularize, and can realize efficient extraction and enrichment of trace amounts of zoledronic acid and risedronic acid in complex actual samples, and meet the relevant high-sensitivity detection requirements.
Owner:福州佳宸生物科技有限公司
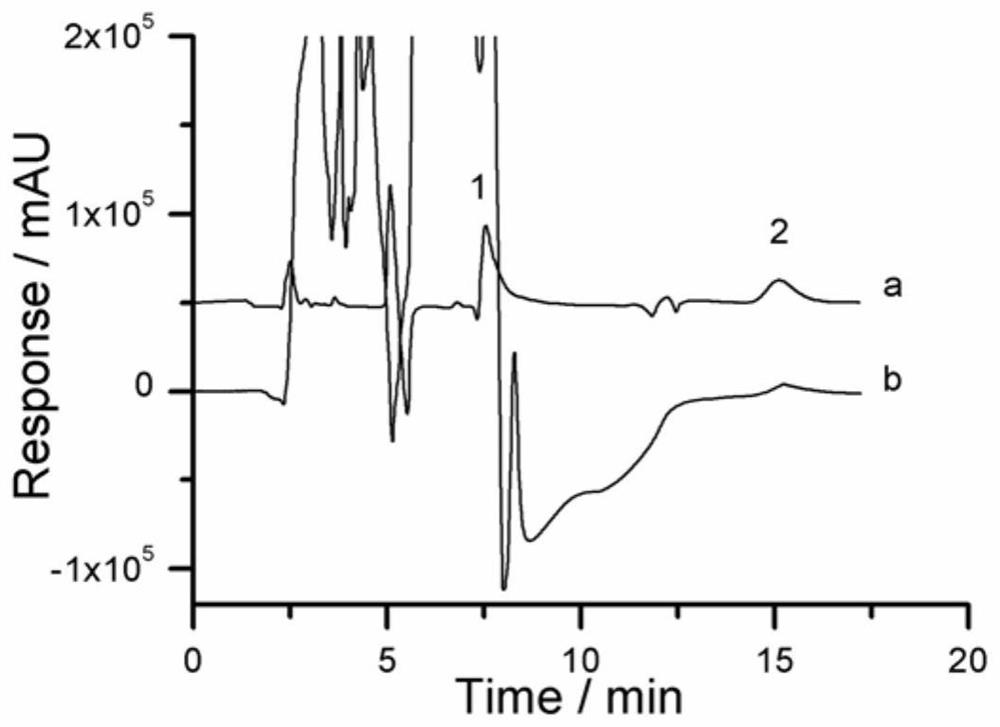
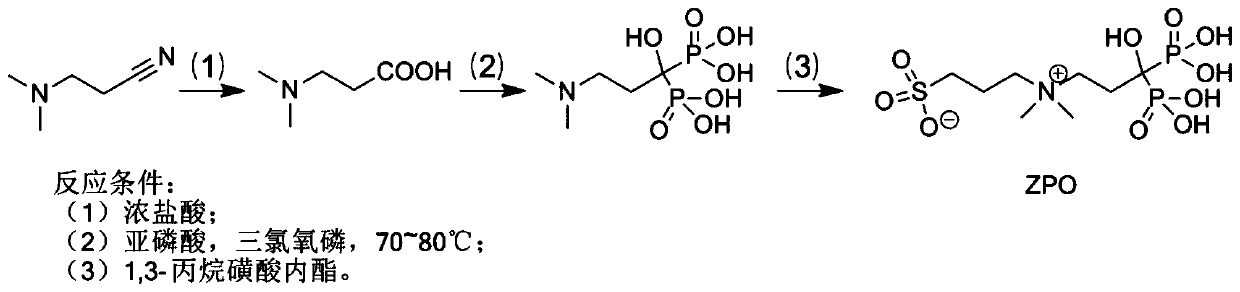
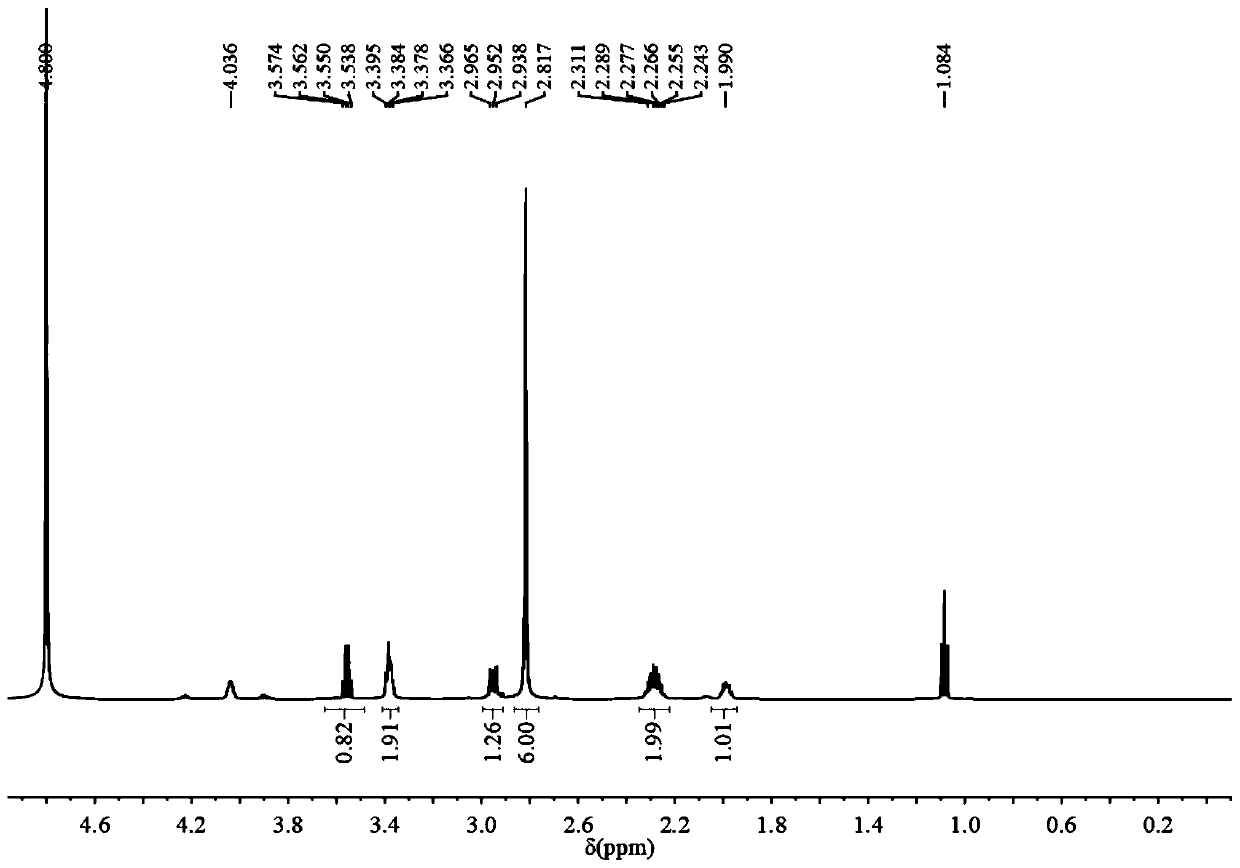
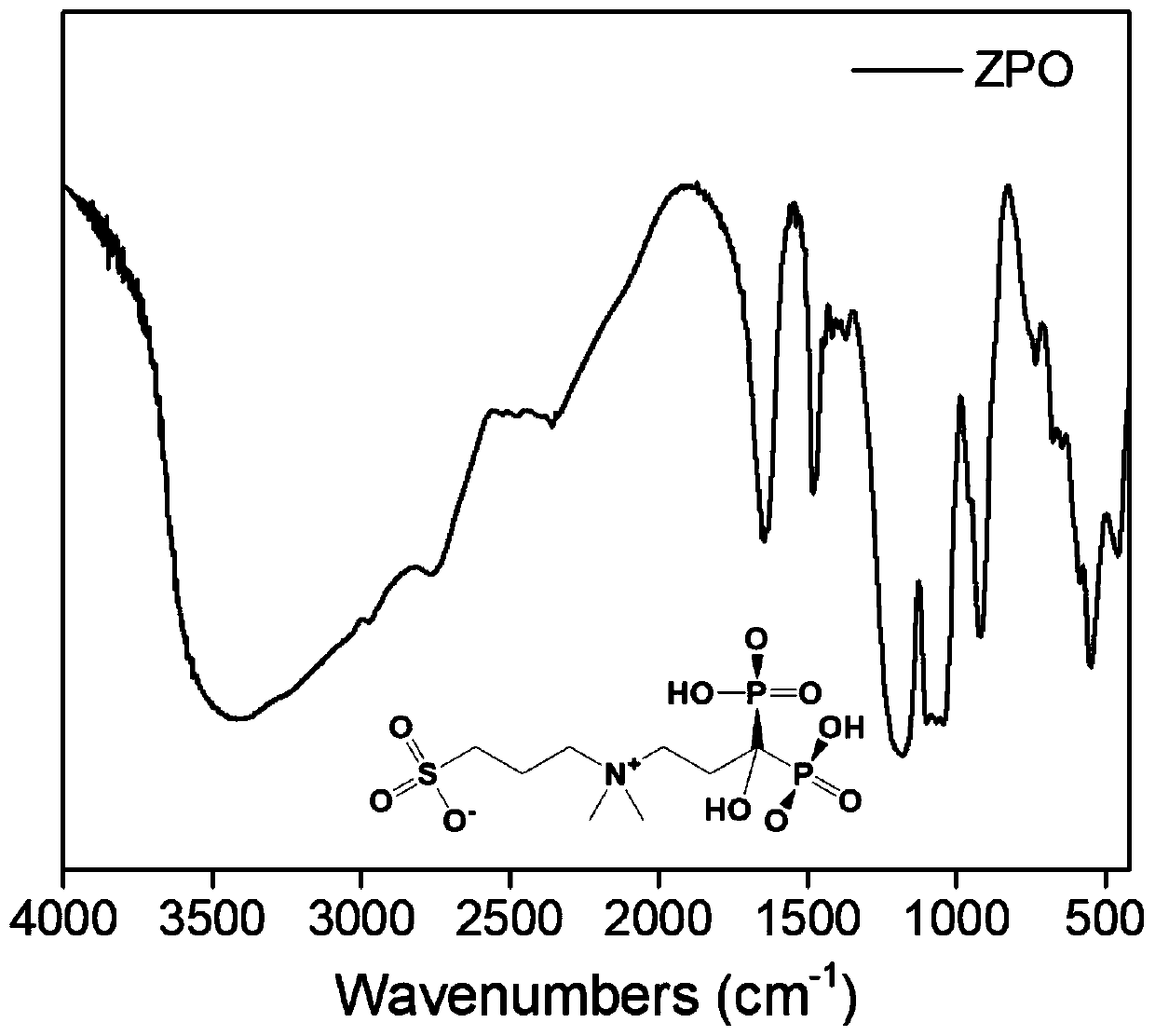
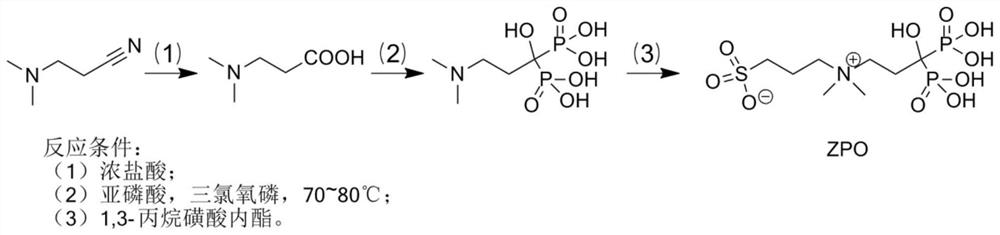
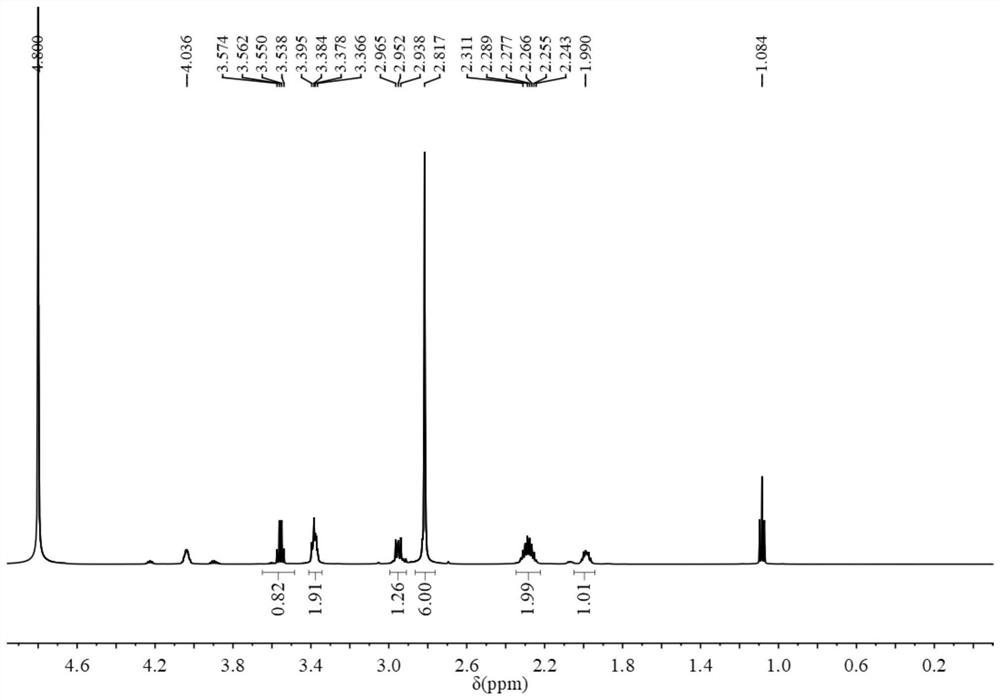
Preparation and application of sulfonic acid-phosphonic acid ligands
ActiveCN110734460AImprove hydrophilicityGroup 5/15 element organic compoundsAntineoplastic agentsBetaineCombinatorial chemistry
The invention relates to preparation and application of a sulfonic acid-phosphonic acid ligand. The structure of the sulfonic acid-phosphonic acid ligand comprises sulfonic acid, quaternary ammonium and phosphonic acid groups, and specifically comprises a sulfonic acid betaine-phosphonic acid ligand, a sulfonic acid-zoledronic acid ligand and a sulfonic acid-risedronic acid ligand. The molecular formula of the sulfobetaine-phosphonic acid ligand is C8H21NO10P2S, and the molecular weight of the sulfobetaine-phosphonic acid ligand is 384.2. The molecular formula of the sulfonic acid-zoledronic acid ligand is C8H16O10N2P2S, and the molecular weight of the sulfonic acid-zoledronic acid ligand is 394.23. The molecular formula of the sulfonic acid-risedronate ligand is C10H17O10NP2S, and the molecular weight of the sulfonic acid-risedronate ligand is 405.25. The sulfonic acid-phosphonic acid ligand disclosed by the invention has excellent hydrophilicity and can be coordinated with various metal elements. The molecules have important applications in the following two aspects: 1, the sulfonic acid-phosphonic acid ligand is used for modifying a hydrophobic nano material, so that the hydrophilicity of the material is remarkably improved, and the sulfonic acid-phosphonic acid ligand can be dispersed in a water phase; and 2, a certain inhibition effect is achieved on tumors.
Owner:SOUTH CHINA NORMAL UNIVERSITY
Solid phase microextraction-high performance liquid chromatography online combined detection method for zoledronic acid and risedronic acid
ActiveCN110568114AImprove specific extraction abilityIncrease coverageComponent separationSolid sorbent liquid separationSolid-phase microextractionElectrostatic interaction
The invention discloses a solid phase microextraction-high performance liquid chromatography online combined detection method for zoledronic acid and risedronic acid. First, a hydroxyapatite functionalized monolithic column is prepared by means of in-situ mineralization, and then, a solid-phase microextraction-high performance liquid chromatography online combined detection method for zoledronic acid and risedronic acid is established by taking the monolithic column as a solid phase microextraction monolithic column and adopting a solid phase microextraction-high performance liquid chromatography online combined system. Efficient extraction and enrichment of zoledronic acid and risedronic acid are achieved through specific electrostatic interaction between hydroxyapatite on the surface ofthe monolithic column and a P-C-P structure carried by an analysis object, and the interference of impurities in a sample matrix on analysis and detection is eliminated. The method is simple, ingenious in process, high in popularization degree of required instruments and easy to popularize, can achieve efficient extraction and enrichment of trace zoledronic acid and risedronic acid in complex actual samples, and meets related high-sensitivity detection requirements.
Owner:福州佳宸生物科技有限公司
Pharmaceutical compositions comprising bisphosphonate derivatives and high-dose cholecalciferol
InactiveCN103191138AImprove complianceExpected synergiesOrganic active ingredientsSkeletal disorderHigh dosesCholecalciferol
The present invention relates to pharmaceutical compositions for preventing or treating osteoporosis, which comprise bisphosphonate-based compounds such as risedronic acids or the salts thereof, ibandronic acids or the salts thereof, or the like, and high-dose cholecalciferol, and which are to be administered once a month. The present invention also relates to pharmaceutical compositions for preventing or treating osteoporosis to be administered once a month, comprising: (a) cholecalciferol-containing granules obtained by adsorbing, to microcrystalline cellulose, the solution obtained by dissolving, into ethanol or an aqueous ethanol solution: (i) 24,000 to 50,000 IUs of cholecalciferol; (ii) one or more first stabilizers selected from among tocopheryl acetate, butylated hydroxytoluene and butylated hydroxyanisole; and iii) a binder; (b) mannitol as a second stabilizer; and (c) risedronic acids or the salts thereof or ibandronic acids or the salts thereof.
Owner:HANLIM PHARMA CO LTD
Preparation and application of a class of sulfonic acid-phosphonic acid ligands
ActiveCN110734460BImprove hydrophilicityGroup 5/15 element organic compoundsAntineoplastic agentsPolymer scienceBetaine
The present invention relates to the preparation and application of a class of sulfonic acid-phosphonic acid ligands. The sulfonic acid-phosphonic acid ligand structure includes sulfonic acid, quaternary ammonium and phosphonic acid groups, and specifically includes sulfobetaine-phosphonic acid ligands, sulfonic acid-zoledronic acid ligands and sulfonic acid-phosphonic acid ligands Sedronic acid ligand; sulfobetaine-phosphonic acid ligand molecular formula is: C 8 H 21 NO 10 P 2 S, the molecular weight is 384.2; the molecular formula of the sulfonic acid-zoledronic acid ligand is: C 8 H 16 O 10 N 2 P 2 S, the molecular weight is 394.23; the molecular formula of the sulfonic acid-risedronic acid ligand is: C 10 H 17 O 10 NP 2 S, the molecular weight is 405.25. The sulfonic acid-phosphonic acid ligand of the invention has excellent hydrophilicity and can coordinate with various metal elements. Such molecules have important applications in the following two aspects: first, to modify hydrophobic nanomaterials, thereby significantly improving the hydrophilicity of the materials and making them dispersible in the aqueous phase; second, to have a certain inhibitory effect on tumors.
Owner:SOUTH CHINA NORMAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com