Preventive agent or therapeutic agent for disease caused by abnormal bone metabolism
A technology for abnormal bone metabolism and preventive medicine, which can be used in bone diseases, anti-tumor drugs, drug combinations, etc., and can solve problems such as difficult to find better treatment methods or preventive methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
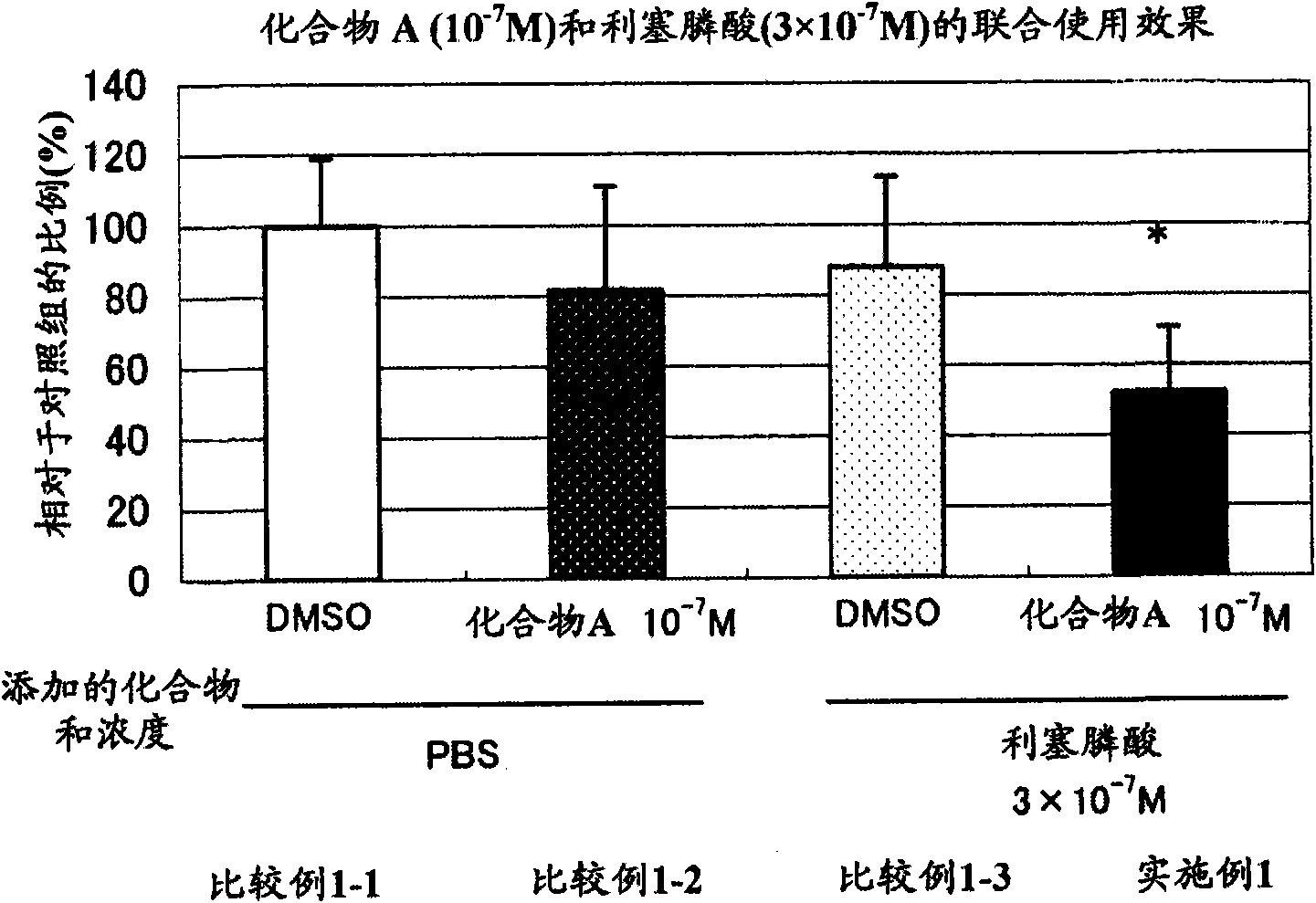
[0042] [Example 1] N-hydroxyl-4-{5-[4-(5-isopropyl-2-methyl-1,3-thiazol-4-yl)phenoxy]pentyloxy}-benzamidine Effects of and risedronic acid on the formation of osteoclast-like cells
[0043] As for the cells from mouse bone marrow, the bone marrow of mouse femur and tibia was collected, and then red blood cells were removed by conventional methods, and 4×10 5 The density of cells / well was seeded in a 96-well plate. After culturing overnight, 1α, 25-dihydroxyvitamin D3 (hereinafter referred to as 1α, 25(OH) 2 D. 3 ) medium to make 1α, 25(OH) 2 D. 3 reach 10 -9 Concentration of M, in 1α, 25(OH) 2 D. 3 In the presence of 7 days, multinucleated osteoclast-like cells were formed. As the medium, αMEM medium supplemented with 10% fetal calf serum (hereinafter referred to as 10% FCS-αMEM) was used. Addition of 1α,25(OH) to cells from mouse bone marrow 2 D. 3 , to the cell culture system from mouse bone marrow: add solvent only (comparative example 1-1), add N-hydroxy-4-{5-[4...
Embodiment 2
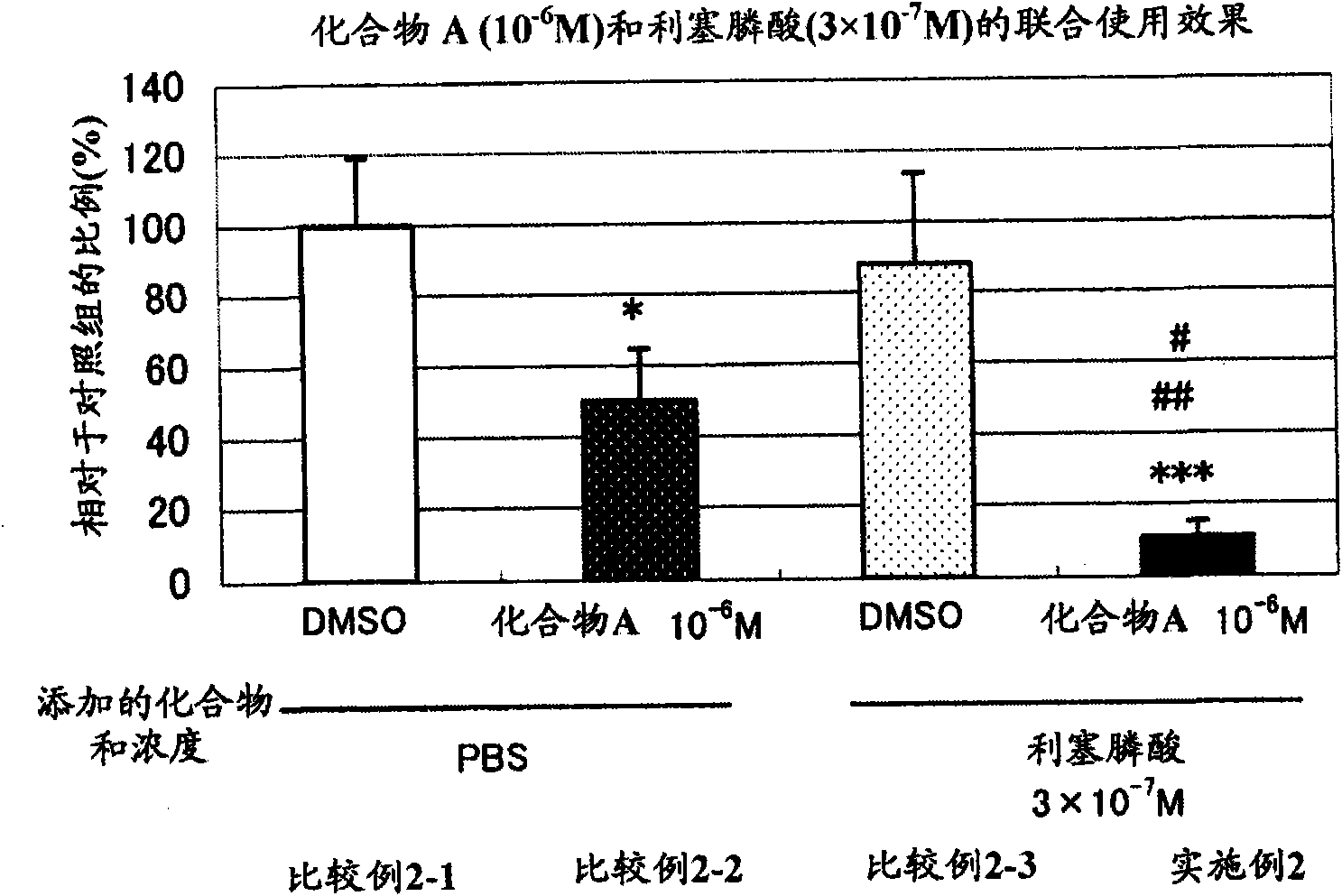
[0058] [Example 2] N-hydroxyl-4-{5-[4-(5-isopropyl-2-methyl-1,3-thiazol-4-yl)phenoxy]pentyloxy}-benzamidine Study on the Effect of And Risedronic Acid on Osteoclast Formation
[0059] In addition to making N-hydroxy-4-{5-[4-(5-isopropyl-2-methyl-1,3-thiazol-4-yl)phenoxy]pentyloxy}-benzamidine in the medium Concentration in 10 -6 Except for M, the same experiment as in Example 1 was performed, and the following groups were set.
Embodiment 3
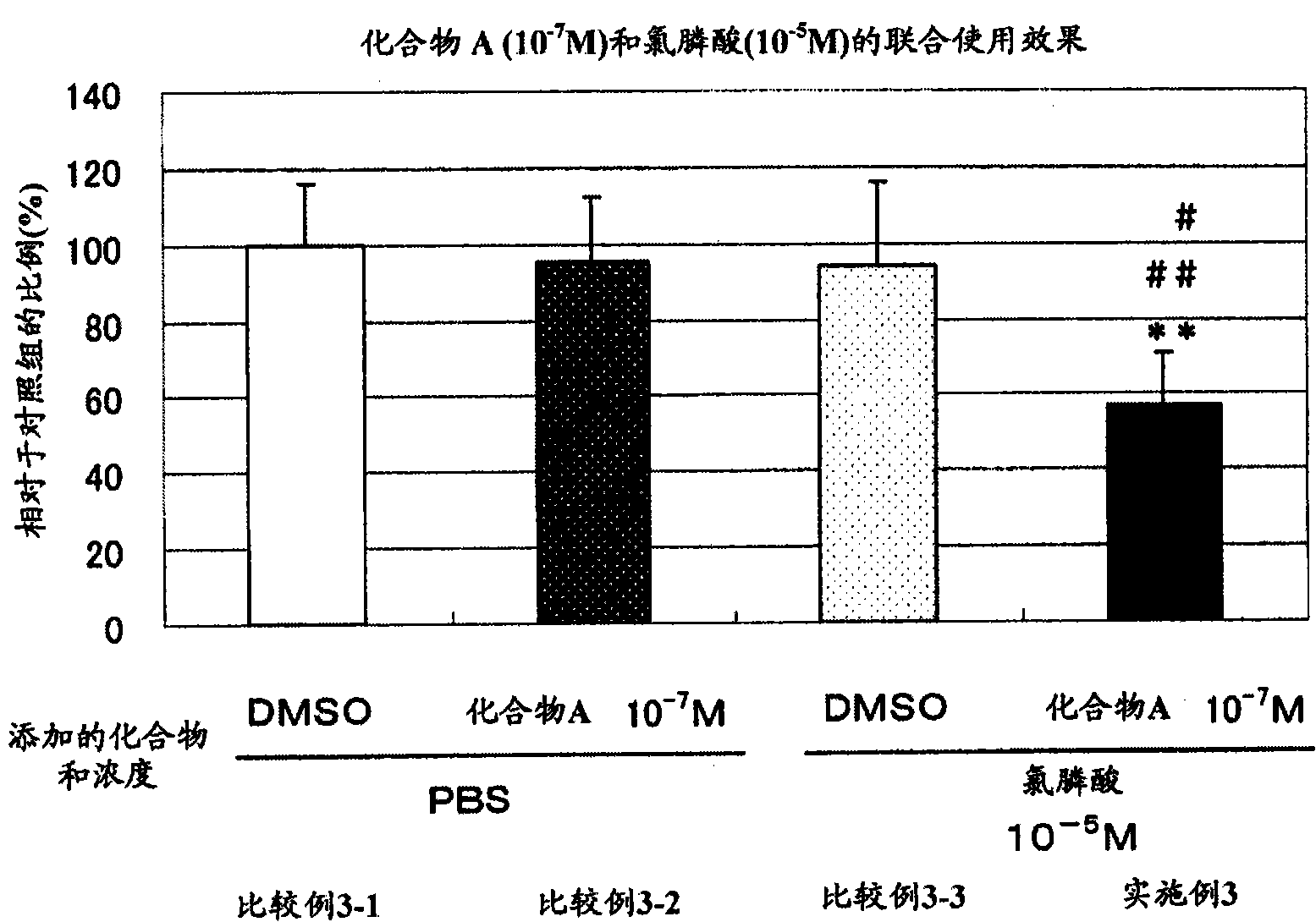
[0071] [Example 3] N-hydroxyl-4-{5-[4-(5-isopropyl-2-methyl-1,3-thiazol-4-yl)phenoxy]pentyloxy}-benzamidine Study on the effect of and clodronic acid on the formation of osteoclasts
[0072] In embodiment 1, except making clodronic acid in comparative example, embodiment with 10 -5 The concentration of M interacts with the cells to replace risedronic acid at 3×10 -7 Except for the concentration of M and the effect on cells, the same experiment as in Example 1 was carried out, and the following groups were set.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com