Preparation and application of sulfonic acid-phosphonic acid ligands
A sulfonic acid and ligand technology, which is applied in the field of preparation of sulfonic acid-phosphonic acid ligands, and can solve the problems of enlargement, limited application range of phase inversion ligands, large molecular weight and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
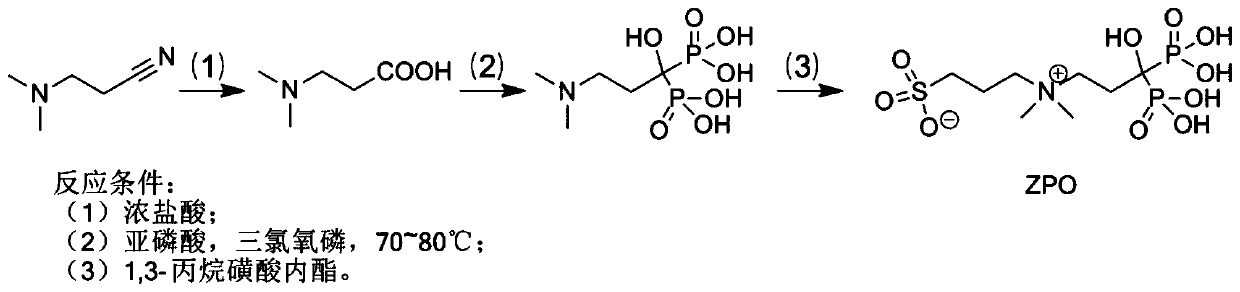
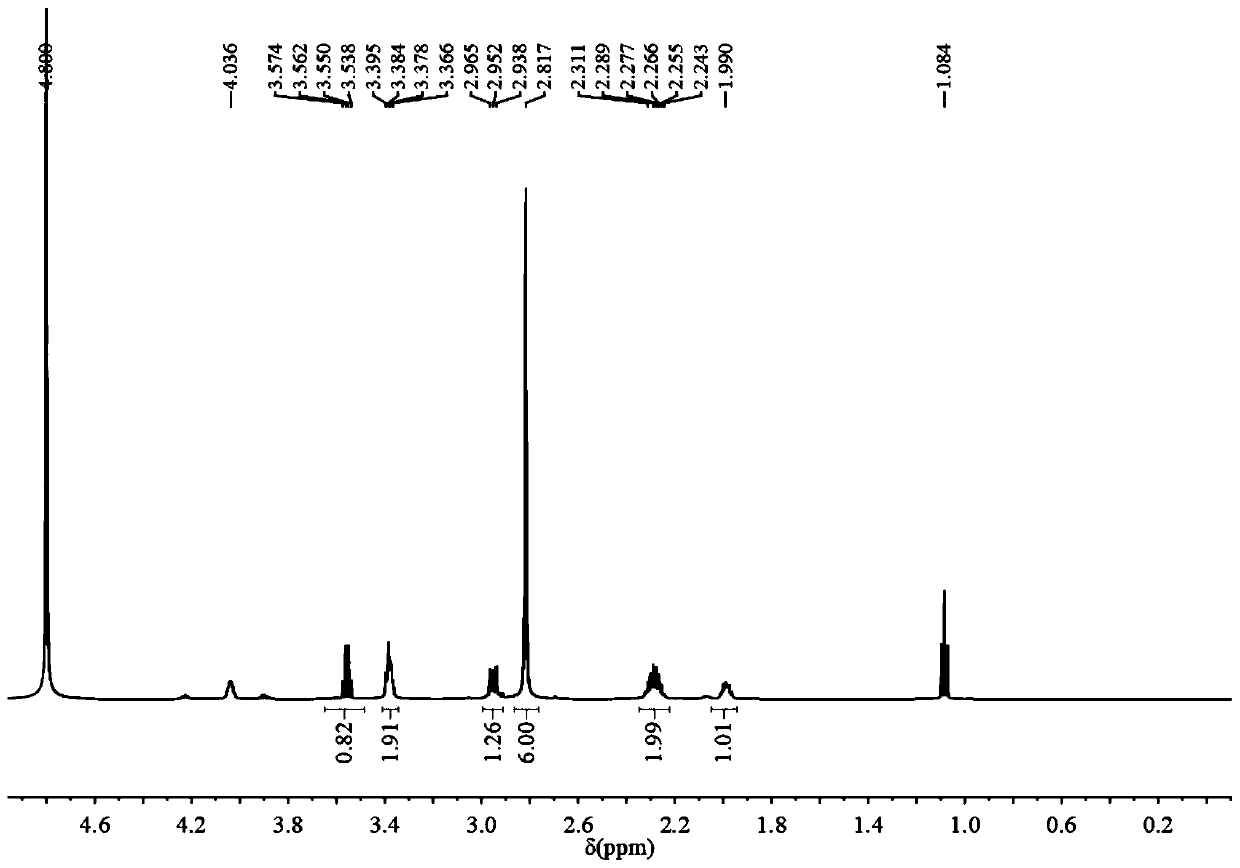
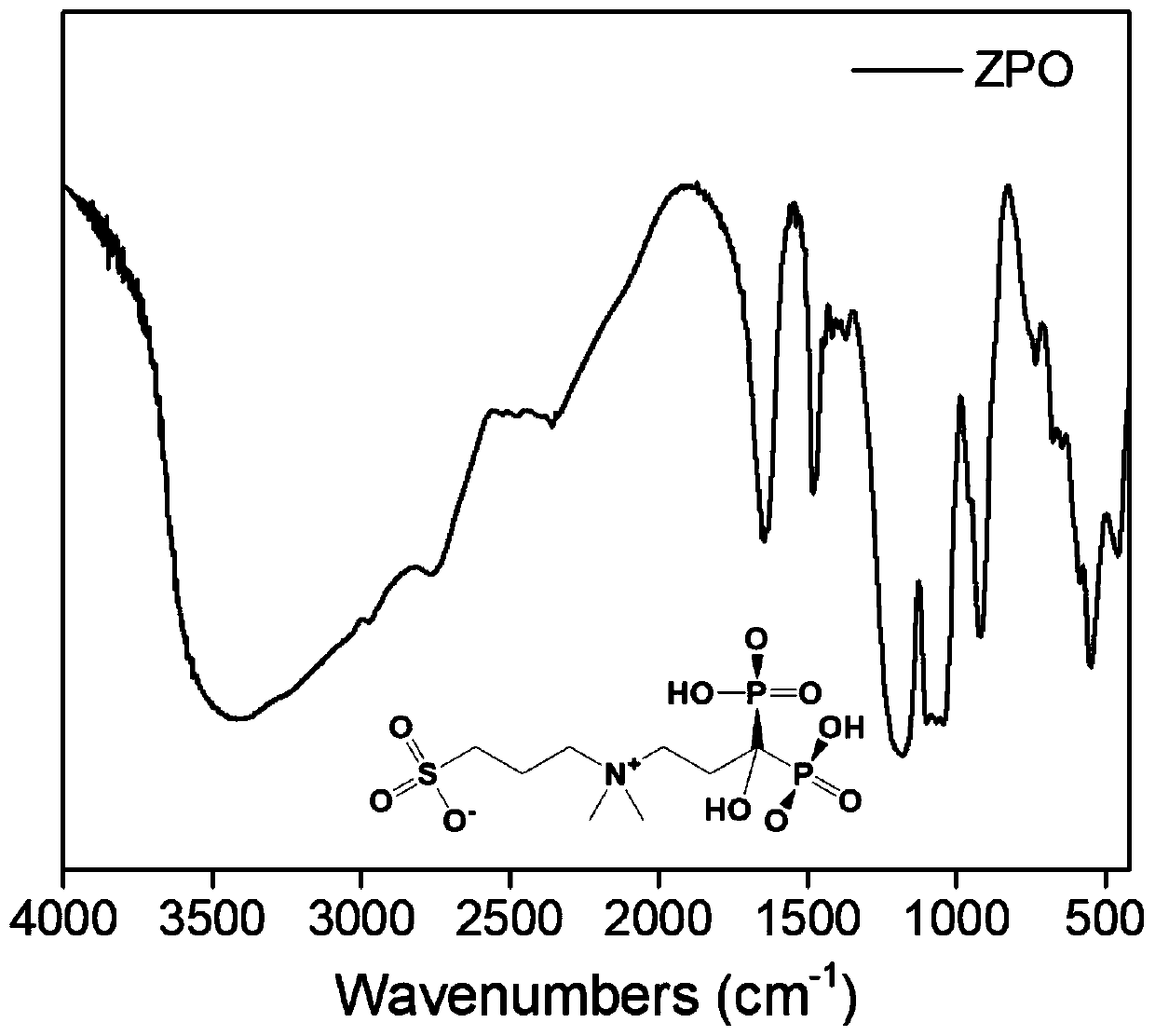
[0078] Embodiment 1: the preparation of ZPO
[0079] (1) Preparation of N,N-dimethylaminopropionic acid
[0080] Add 20.2g of N,N-dimethylpropionitrile into a 250mL round bottom flask, add 100mL of concentrated hydrochloric acid dropwise, then stir at room temperature for 3h, wait for the solution to cool to room temperature, filter the solution, concentrate the filtrate under reduced pressure, add 40mL After washing twice with isopropanol, a white precipitate, namely N,N-dimethylalanine, can be obtained.
[0081] (2) Preparation of 3-dimethylamino-1-hydroxyl-1,1-propane diphosphonic acid
[0082] Mix 7.6g of N,N-dimethylalanine and 15.7g of phosphorous acid into a 100mL round bottom flask, and heat and stir at 75°C for 30min. Slowly add 18.4g of phosphorus oxychloride dropwise, finish dropping within 1h, heat to 80°C, and reflux for 5h. After the solution was cooled to room temperature, water was slowly added to quench the reaction, then stirred at room temperature for 3 h...
Embodiment 2
[0085] Embodiment 2: the preparation of ZOL
[0086] Add 0.5 g of zoledronic acid to 50 mL of ethanol, and then add 1.2 mL of methanol solution containing 25% tetramethylammonium hydroxide to completely dissolve the zoledronic acid. 1,3-Propane sultone (0.269g) dissolved in 5mL of ethanol was added dropwise to the zoledronic acid solution, and reacted at room temperature for 24 hours, then the solvent was removed by rotary evaporation, and 1.2mL of concentrated hydrochloric acid and 50mL of ethanol, the precipitate was precipitated, washed several times with ethanol, and dried to obtain the sulfonic acid-zoledronic acid. ZOL's 1 H NMR picture as Figure 5 shown.
Embodiment 3
[0087] Embodiment 3: the preparation of LSL
[0088] Add 0.52 g of risedronic acid into 50 mL of ethanol, and then add 1.2 mL of methanol solution containing 25% tetramethylammonium hydroxide to completely dissolve risedronic acid. Add 1,3-propane sultone (0.269 g) dissolved in 5 mL of ethanol dropwise into the risedronic acid solution, and react at room temperature for 24 hours, then remove the solvent by rotary evaporation, add 1.2 mL of concentrated hydrochloric acid and 50 mL of ethanol, the precipitate was precipitated, washed several times with ethanol, and dried to obtain the sulfonic acid-risedronic acid. LSL's 1 H NMR picture as Figure 7 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com