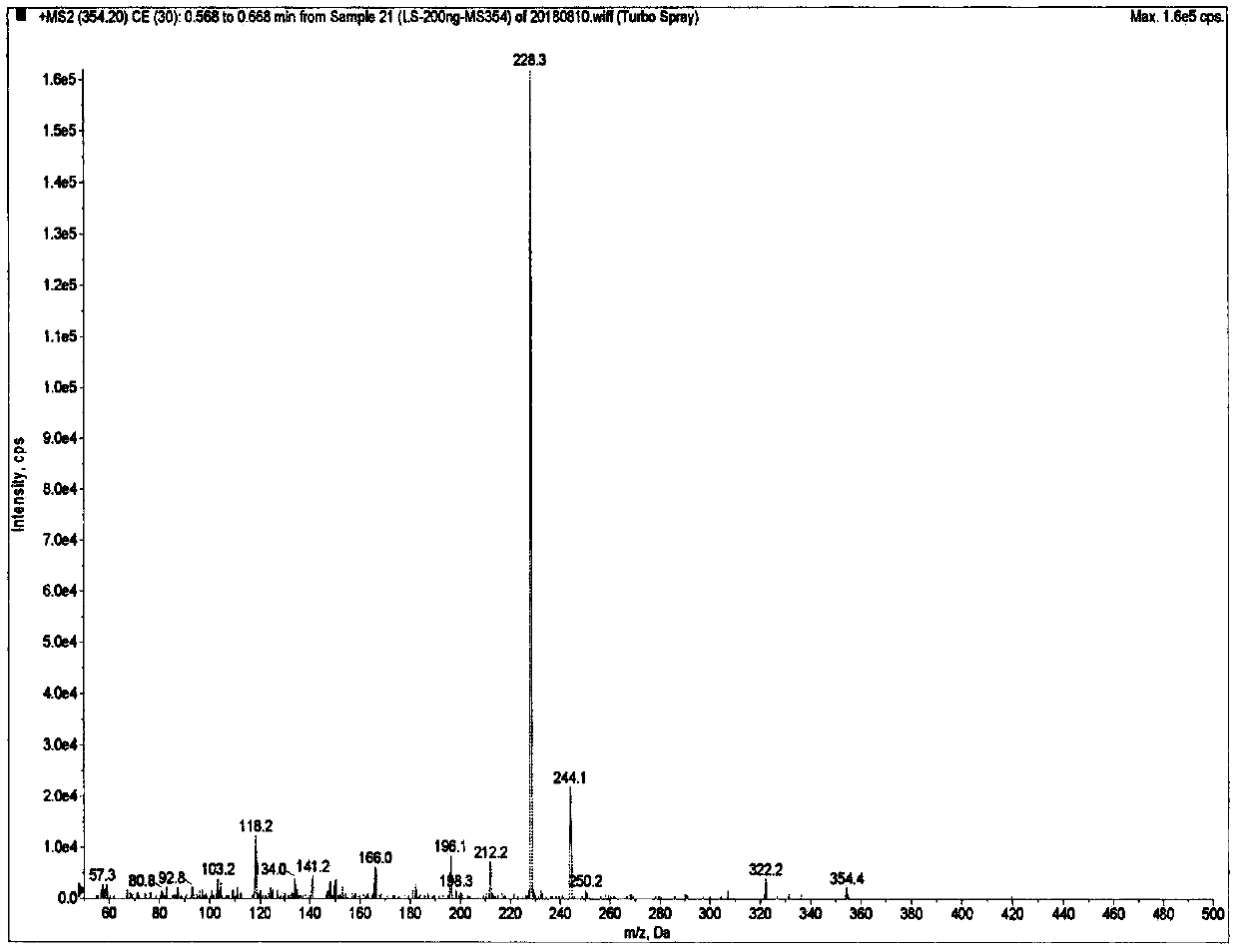
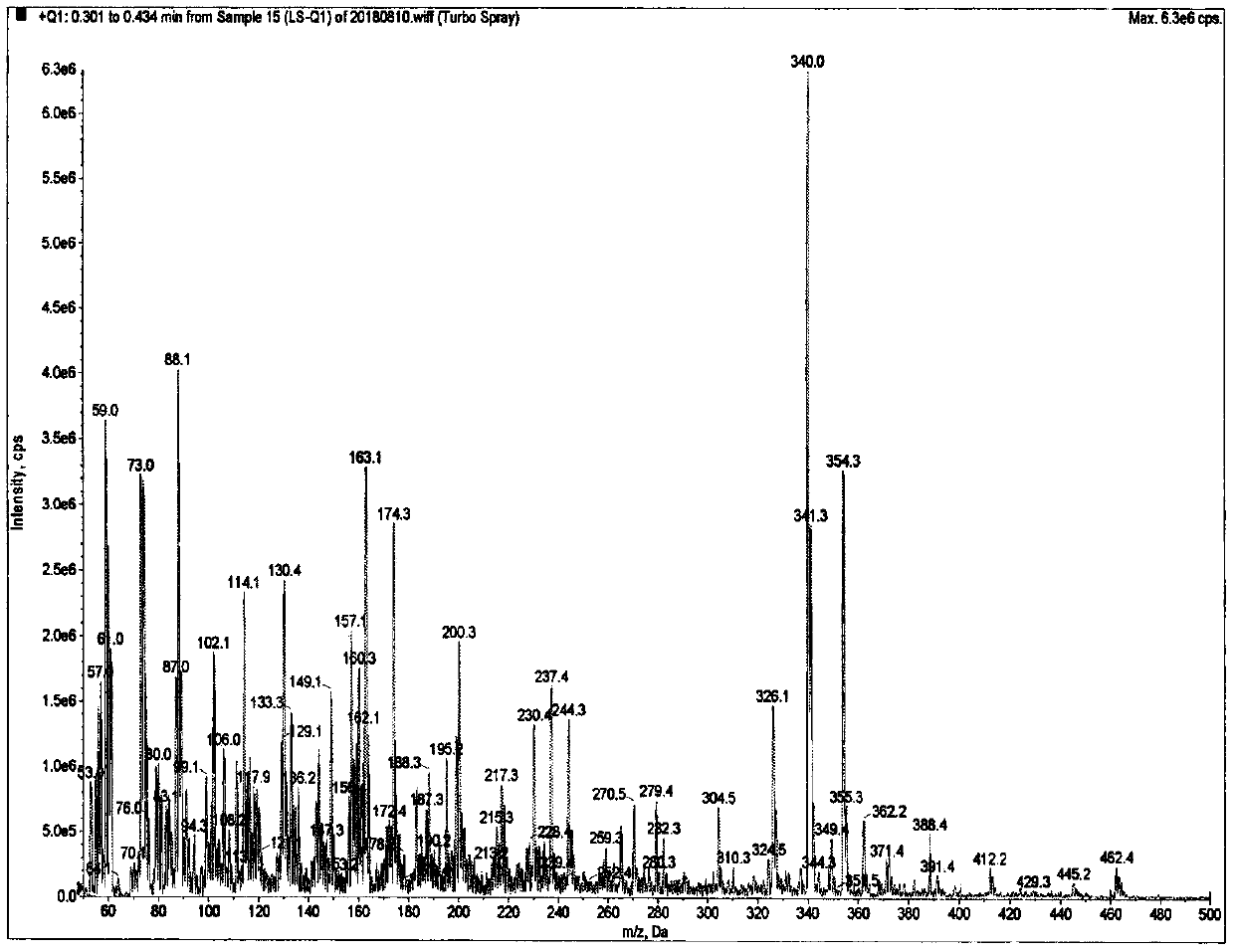
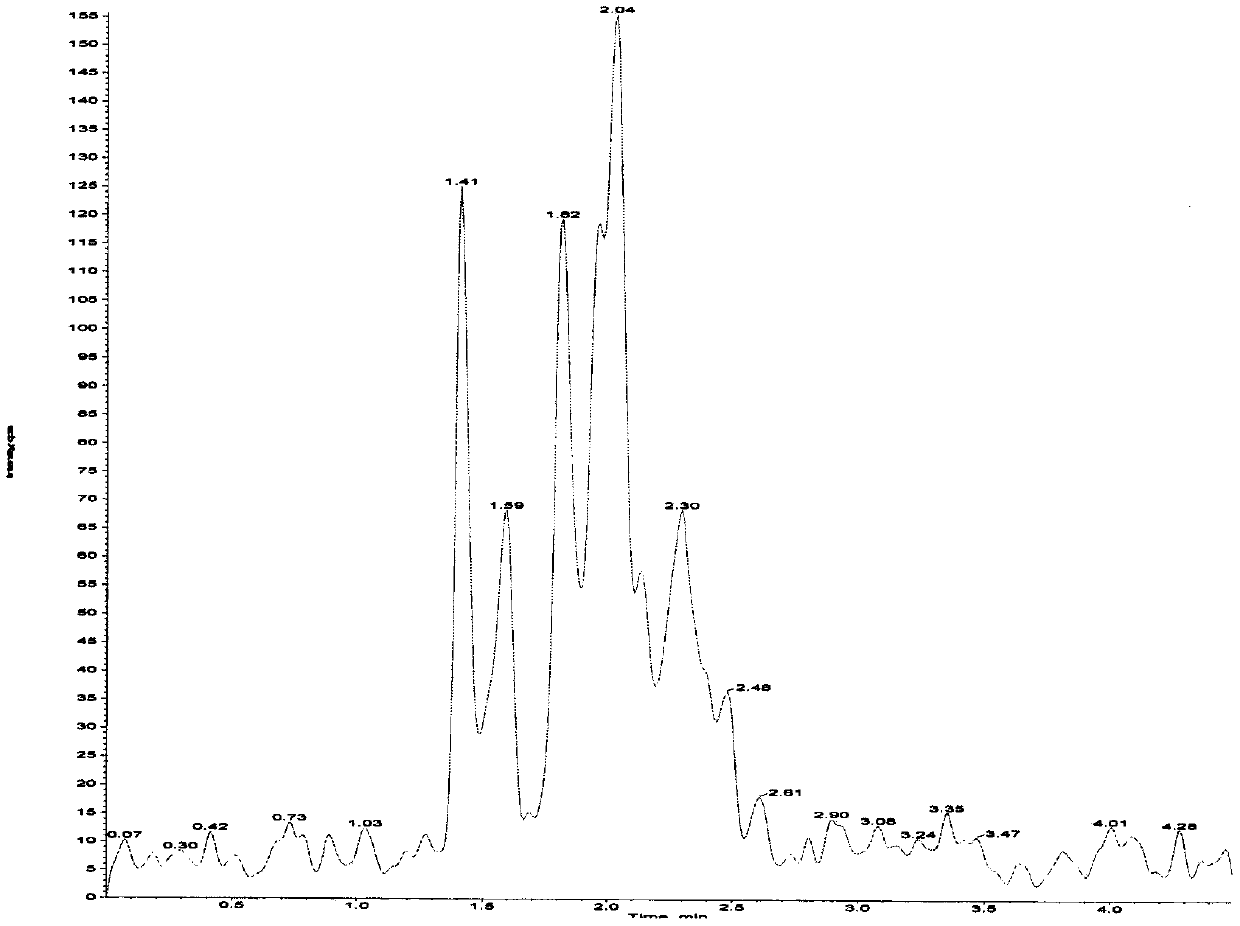
Method of determining risedronate in human plasma based on LC-MS/MS pre-derivatization and application thereof
A risedronic acid and derivatization technology, which is applied in the field of drug analysis, can solve the problems affecting the development of bisphosphonic acid drugs, affecting the derivatization efficiency, poor reproducibility, etc., and achieves simplified sample pretreatment process, short running time and high sensitivity. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1. Drugs and reagents
[0027] Risedronate sodium reference substance (batch number: 100613-201402, content 87.3%) was purchased from China National Institutes for Food and Drug Control, internal standard risedronic acid-D4 (batch number: 1287-010A1, content 99.2%) was purchased from Canada TLC Pharmaceutical Standards Ltd, derivatization reagent Trimethylsilyldiazomethane was purchased from Shanghai Aladdin Biotechnology Co., Ltd. Chromatographically pure methanol and acetonitrile were purchased from Honeywell China Co., Ltd., analytically pure formic acid was purchased from Fisher Co., Ltd., analytically pure trichloro Acetic acid was purchased from Beijing Chemical Plant, analytically pure ammonium acetate was purchased from Shanghai Aladdin Biotechnology Co., Ltd., ultrapure water was prepared by MilliQ pure water instrument of Merck, Germany; blank plasma was provided by the clinical pharmacology base.
[0028] 2. Instruments and conditions
[0029] The liquid chr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com