Method for detecting buspirone and metabolites thereof through LC_MS_MS
A metabolite, buspirone technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve problems such as imperfections, achieve the effects of small sample size, short time, and improved detection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1 Detection of buspirone and its metabolites
[0077] 1. Preparation of standard working solution
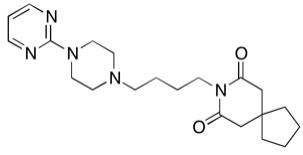
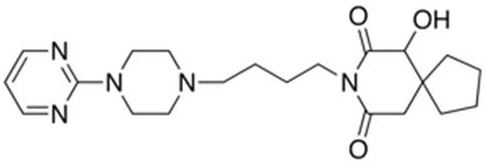
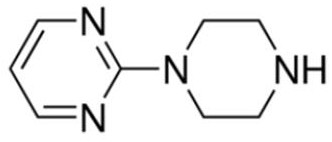
[0078] 1. Prepare standard solutions containing buspirone, 6-hydroxybuspirone and 1-(2-pyrimidinyl)-piperazine. The concentrations of the three targets are 1ng / mL and 4ng / mL , 10ng / mL, 40ng / mL, 100ng / mL, 200ng / mL and 400ng / mL seven serial standard solutions.
[0079] 2. Prepare internal standard solution: Mixed isotope internal standard solution of buspirone-d8, 6-OH-buspirone-d8 and 1-(2-pyrimidinyl)-piperazine-d8 with a concentration of 40ng / mL standard solution.
[0080] 3. Take seven centrifuge tubes, add 10 μL of the above-mentioned seven different concentrations of standard solutions, add 10 μL of mixed isotope internal standard solution, add methanol:water (V:V)=70:30 mixed solvent 100 μL and mix well. Get seven standard working solutions with different concentrations for later use.
[0081] 2. Pretreatment of blood samples
[0082] 1. Take 2 mL of the...
Embodiment 2
[0133] Embodiment 2 Determination of limit of quantification and limit of detection
[0134] A blank serum sample containing no target substance was used to add a standard to prepare a standard containing buspirone (0.1ng / mL), 6-hydroxybuspirone (0.1ng / mL) and 1-(2-pyrimidinyl)- Piperazine (0.1ng / mL) blood samples were diluted to different degrees with blank serum samples to prepare blood samples diluted 2 times, 3 times, 4 times, and 5 times respectively, and 10 μL of mixed isotope internal standard solution was added. Subsequent processing is the same as the above blood sample pretreatment process, and the determination is carried out according to the LC_MS_MS detection conditions determined above. The limits of detection and quantitation for buspirone, 6-hydroxybuspirone, and 1-(2-pyrimidinyl)-piperazine are shown below:
[0135] Buspirone:
[0136] (1) Limit of detection (LOD): 0.02ng / mL, S / N=5.4.
[0137] (2) Limit of quantification (LOQ): 0.03ng / mL, S / N=14.6.
[0138...
Embodiment 3
[0145] Embodiment 3 The mensuration of rate of recovery and precision
[0146] The standard working solutions of buspirone, 6-hydroxybuspirone and 1-(2-pyrimidinyl)-piperazine were prepared respectively to concentrations of 0.4 ng / mL, 4.0 ng / mL, 20 ng / mL and 32 ng 4 kinds of concentrations / mL carry out sample addition recovery rate and precision experiment, measure by the LC_MS_MS method among the embodiment 1, measure 4 samples in parallel, buspirone, 6-hydroxybuspirone, 1-(2- The recovery and precision of pyrimidinyl)-piperazine are shown in Table 7.
[0147] Table 7 Buspirone, 6-hydroxybuspirone, 1-(2-pyrimidinyl)-piperazine spiked recoveries
[0148]
[0149] It can be seen from Table 7 that the precision of buspirone, 6-hydroxybuspirone and 1-(2-pyrimidinyl)-piperazine in the concentration range of 4 different levels is 0.61% ~ 2.63%, and the average recovery The rate is 99.50% ~104.75%, the precision is high, and the recovery rate of the standard addition is good, so ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com