Preparation of risedronate zinc micro-nano adjuvant and application of adjuvant as vaccine adjuvant
A technology of zinc risedronate and risedronate, applied in the field of medicine, can solve the problems of unsatisfactory antigen immunity enhancement, poor tolerance, weak activity of aluminum adjuvant and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
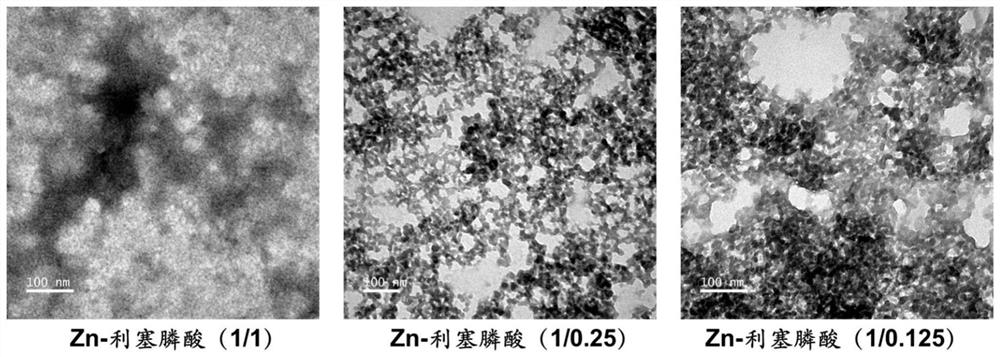
[0085] Preparation Example 1: Preparation of Zinc Risedronate Adjuvant (Zn-Risedronic Acid (1 / 0.25))
[0086] Risedronate Sodium (C 7 h 10 NNaO 7 P 2 ): purchased from Hunan Huateng Pharmaceutical Co., Ltd.
[0087] Anhydrous Zinc Chloride (ZnCl 2 ): purchased from Xilong Chemical
[0088] Disodium hydrogen phosphate dodecahydrate (Na 2 HPO 4 12H 2 O): purchased from Xilong Chemical Industry
[0089] Sodium hydroxide (NaOH): purchased from Xilong Chemical Industry
[0090] Solution preparation:
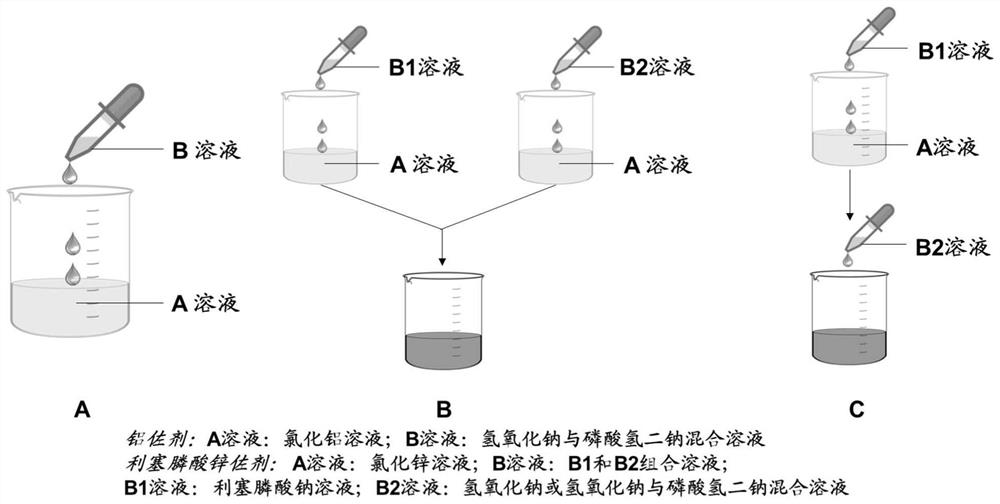
[0091] According to the Zn / risedronic acid molar concentration ratio of 1:0.25, prepare 50mL of 31.11mM zinc chloride solution, defined as solution A; prepare 50mL (7.78mM risedronic acid+36mM sodium hydroxide+15.55mM dihydrogen phosphate Sodium) solution is defined as B solution, and A solution and B solution are filtered with a 0.22 μm filter membrane for subsequent use.
[0092] Preparation of Zn-risedronic acid (1 / 0.25) adjuvant suspension and determination of physic...
preparation example 2
[0095] Preparation Example 2: Preparation of Zinc Risedronate Adjuvant (Zn-Risedronic Acid (1 / 1))
[0096] Reagent sources refer to Preparation Example 1.
[0097] Solution preparation:
[0098] According to the Zn / risedronic acid molar concentration ratio of 1:1, prepare 50mL 31.11mM zinc chloride solution, which is defined as solution A; prepare 50mL (31.11mM risedronic acid + 60mM sodium hydroxide) solution, which is defined as B solution, solution A and solution B were filtered with a 0.22 μm filter membrane for later use.
[0099] Preparation of Zn-risedronic acid (1 / 1) adjuvant suspension and determination of physical and chemical properties:
[0100]Please refer to Preparation Example 1.
preparation example 3
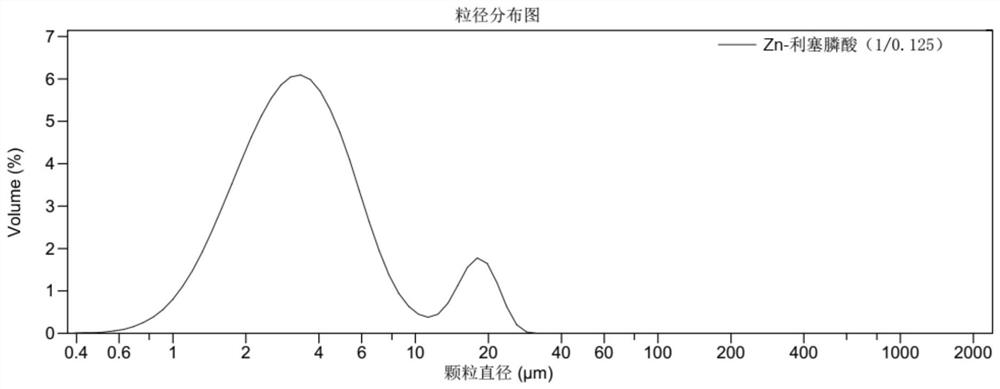
[0101] Preparation Example 3: Preparation of Zinc Risedronate Adjuvant (Zn-Risedronic Acid (1 / 0.125))
[0102] Reagent sources refer to Preparation Example 1.
[0103] Solution preparation:
[0104] According to the Zn / risedronic acid molar concentration ratio of 1:0.125, prepare 1L of 124.44mM zinc chloride solution, which is defined as solution A; prepare 1L (15.56mM risedronic acid+60mM sodium hydroxide+184mM disodium hydrogen phosphate ) solution, which is defined as B solution, A solution and B solution are filtered with 0.22 μm filter membrane for later use.
[0105] Preparation of Zn-risedronic acid (1 / 0.125) adjuvant suspension and determination of physical and chemical properties:
[0106] Please refer to Preparation Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com