Carboxyl contained NSAIDS (nonsteroidal anti-inflammatory drugs) salt
An antipyretic and analgesic drug and an antipyretic and analgesic technology are applied in the salt field of carboxyl-containing non-steroidal antipyretic and analgesic drugs, and can solve the problems of water insolubility, large toxic and side effects, drug resistance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
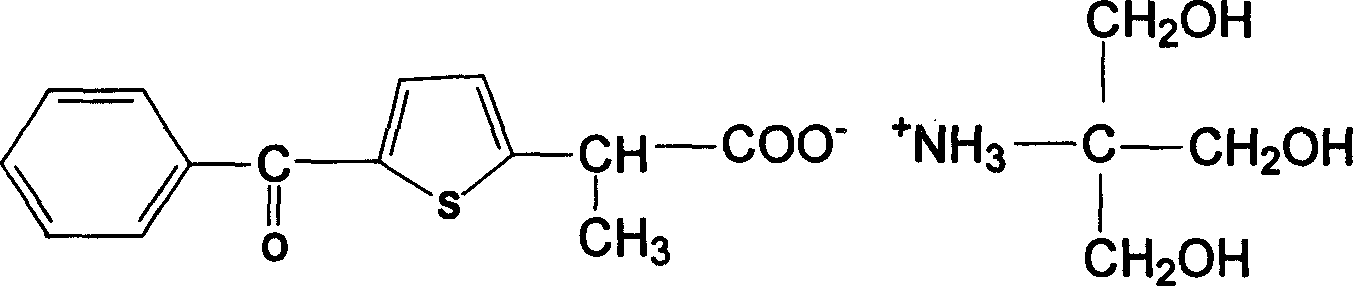
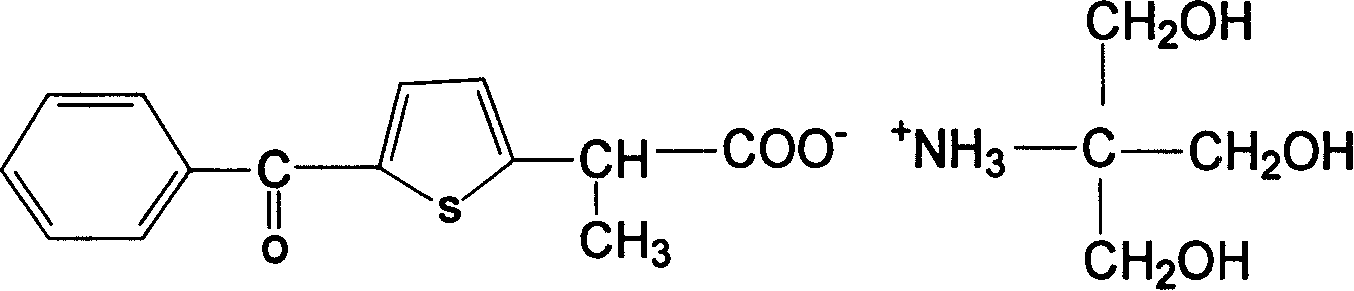
[0016] Embodiment 1: the preparation of saprofen acid tromethamine salt
[0017] In a 500ml dry and clean three-neck flask, add 30g of serprofen acid and 300ml of anhydrous benzene, heat and stir until completely dissolved, add 13g of tromethamine under the state of complete dissolution, heat and reflux the mixture for 3 hours, TLC thin layer identification reaction completely (developing solvent: ethyl acetate: ethanol: glacial acetic acid = 5:5:0.5), slightly cool the mixture and add 200ml of absolute ethanol, put the mixture in the refrigerator to fully cool, filter the precipitated white crystals with suction, and use a small amount of cold The solid was washed with anhydrous ether. Vacuum drying at 80°C to obtain 29g of serprofen acid tromethamine salt, yield 87.9%, mp: 186-188°C, product verified by four major spectra: uv: maximum absorption at 254nm, maximum absorption at 289nm .
Embodiment 2
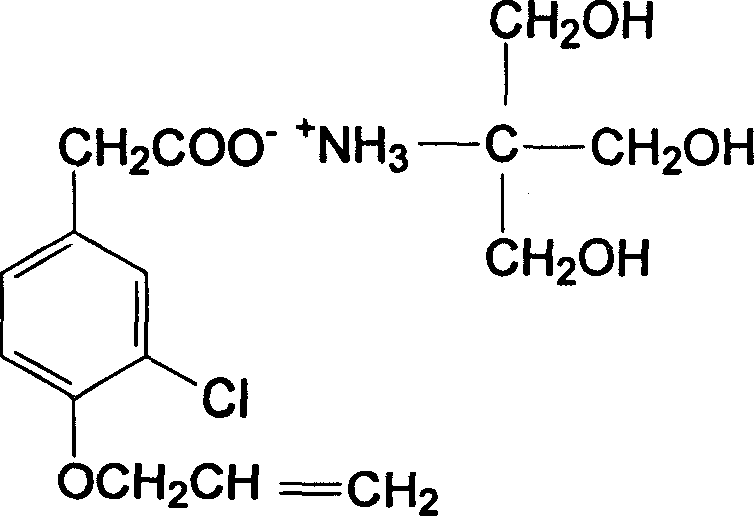
[0018] Embodiment 2: the preparation of aclofenac tromethamine salt
[0019] In a 250ml three-necked flask, add 50g of aclofenac to 200ml of methanol. Under the condition of avoiding light, stir and heat up to 30°C, and the mixture dissolves. At this time, add 38g of trometamol, stir until the reaction is clear, and react for 8 hours , TLC identified that the reaction was complete (developing agent: n-hexane: acetone: glacial acetic acid = 10:5:0.2), after the reaction was completed, 60 ml of ether was added, and the mixture was cooled to obtain a white crystalline powder. The solid was filtered by suction and washed with a small amount of ether. Vacuum drying at 50°C yielded 79g, the yield was 89.8%, mp: 110-111°C, the product was confirmed by four major spectra: UV: maximum absorption at 286nm.
Embodiment 3
[0020] Embodiment 3: Solubility experiment of aclofenac tromethamine salt
[0021] Take aclofenac tromethamine salt respectively as shown in Table 1, add corresponding solvents respectively, shake vigorously for 5 seconds every 5 minutes, observe after 30 minutes, the results are shown in Table 1:
[0022] Table 1: Solubility test of aclofenac tromethamine salt
[0023]
[0024] 0.1mol / ml
[0025] The experimental results show that: after the salt formation of aclofenac and tromethamine, it is easily soluble in water, 0.1mol / ml hydrochloric acid, 0.1mol / ml sodium hydroxide, slightly soluble in acetone and ethanol, and insoluble in ether.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mp | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com