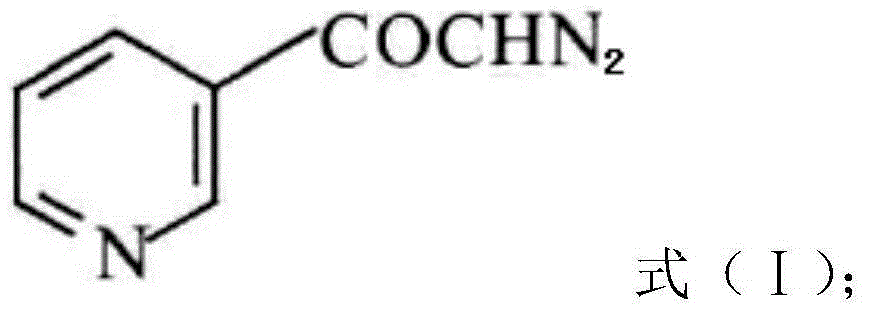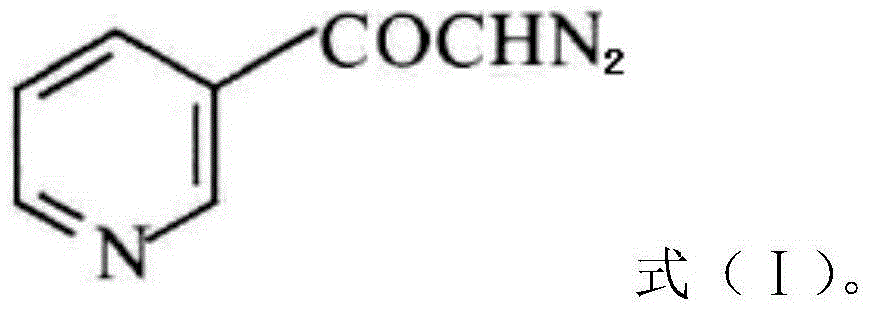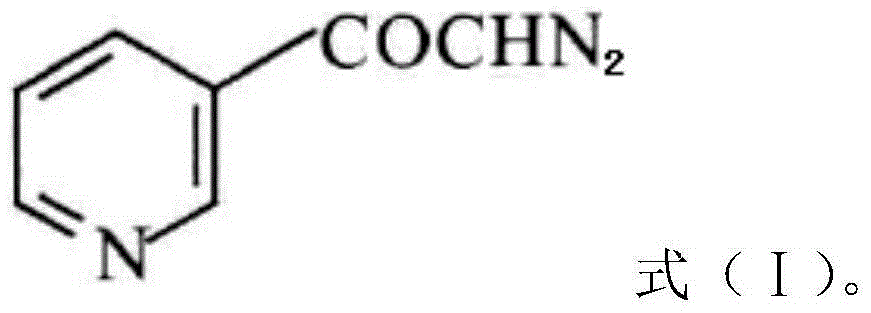Preparation method of risedronate sodium
A technology of risedronate sodium and risedronic acid, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve the problem of long reaction time, harsh reaction conditions, and reaction steps Avoid multiple problems, achieve the effect of avoiding side reactions, high purity, and less loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A preparation method of risedronate sodium, comprising the steps of:
[0025] (1) 3-pyridineformyl chloride in 3-pyridineformyl chloride hydrochloride is freed.
[0026] When the temperature is 15° C., 3-pyridinecarbonyl chloride hydrochloride is added to a mixture of neutral organic solvent and basic organic solvent. The neutral organic solvent is a mixture of methyl tert-butyl ether, butyl acetate and kerosene in a volume ratio of 5:0.5:10; 5 grams of 3-pyridineformyl chloride hydrochloride are added to every 100 mL of the neutral organic solvent Salt. The basic organic solvent is trioctylamine, and the ratio of trioctylamine to 3-pyridinecarbonyl chloride hydrochloride is 6:1. It was then stirred for 30 minutes and filtered. HCl in 3-pyridineformyl chloride hydrochloride and trioctylamine generate trioctylamine hydrochloride, which is precipitated in solid form. The content of 3-pyridineformyl chloride in the filtrate is detected by high performance liquid chroma...
Embodiment 2
[0041] A preparation method of risedronate sodium, comprising the steps of:
[0042] (1) 3-pyridineformyl chloride in 3-pyridineformyl chloride hydrochloride is freed.
[0043] When the temperature is 15° C., 3-pyridinecarbonyl chloride hydrochloride is added to a mixture of neutral organic solvent and basic organic solvent. The neutral organic solvent is a mixture of methyl tert-butyl ether, ethyl acetate and kerosene in a volume ratio of 3:0.3:10; 5 grams of 3-pyridineformyl chloride hydrochloride are added to every 100 mL of the neutral organic solvent Salt. The basic organic solvent is trioctylamine, and the ratio of trioctylamine to 3-pyridinecarbonyl chloride hydrochloride is 6:1. It was then stirred for 30 minutes and filtered. HCl in 3-pyridineformyl chloride hydrochloride and trioctylamine generate trioctylamine hydrochloride, which is precipitated in solid form. The content of 3-pyridineformyl chloride in the filtrate was detected by high performance liquid chrom...
Embodiment 3
[0058] A preparation method of risedronate sodium, comprising the steps of:
[0059] (1) 3-pyridineformyl chloride in 3-pyridineformyl chloride hydrochloride is freed.
[0060] When the temperature is 15° C., 3-pyridinecarbonyl chloride hydrochloride is added to a mixture of neutral organic solvent and basic organic solvent. The neutral organic solvent is a mixture of methyl tert-butyl ether, propyl acetate and kerosene in a volume ratio of 6:0.8:13; 5 grams of 3-pyridineformyl chloride hydrochloride are added to every 100 mL of the neutral organic solvent Salt. The basic organic solvent is trioctylamine, and the ratio of trioctylamine to 3-pyridinecarbonyl chloride hydrochloride is 6:1. It was then stirred for 30 minutes and filtered. HCl in 3-pyridineformyl chloride hydrochloride and trioctylamine generate trioctylamine hydrochloride, which is precipitated in solid form. The content of 3-pyridineformyl chloride in the filtrate was detected by high performance liquid chro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com