Pharmaceutical composition comprising risedronic acid or a salt thereof and vitamin D
A technology of risedronic acid and its composition, which is applied in the field of pharmaceutical compositions containing risedronic acid or its salt and vitamin D, and can solve problems such as difficulty in ensuring content balance, lack of specific disclosure, and low stability of dosage forms , to achieve excellent stability, enhance homeostasis, and inhibit bone resorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 to 5
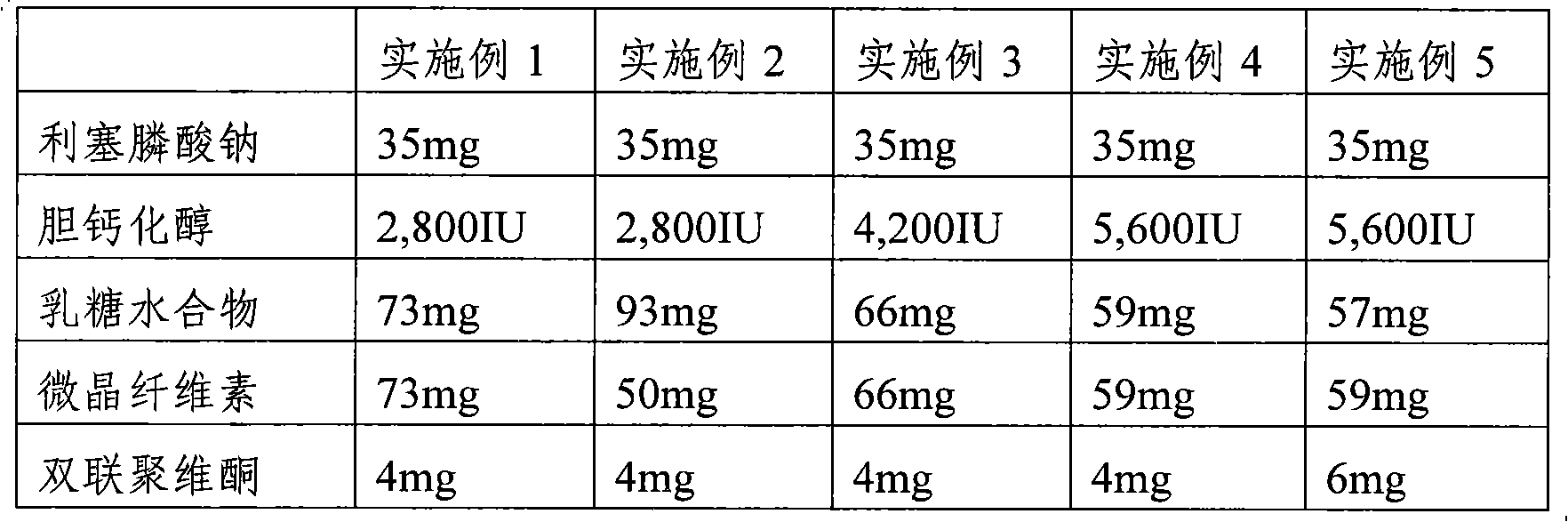
[0031] Tablets containing risedronate sodium and cholecalciferol were prepared according to the ingredient contents in Table 1 below.
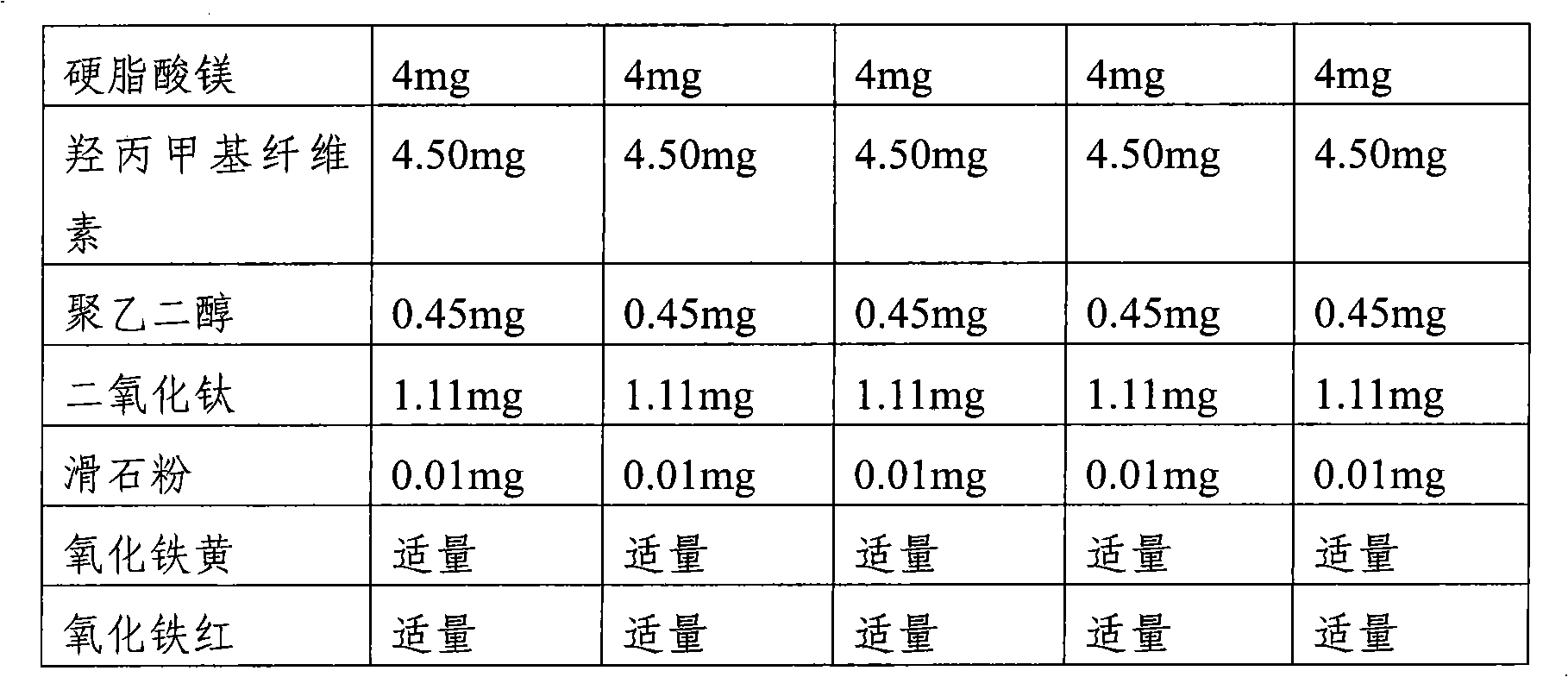
[0032] In a double cone mixer (ERWEKA Gmbh, Germany), risedronate sodium (contents in Table 1 are expressed as anhydrous content), cholecalciferol, lactose monohydrate (lactose monohydrate), microcrystalline cellulose Mix for about 10 minutes. After the crospovidone was added to the above mixture and mixed for about 5 minutes, the magnesium stearate was added and mixed for about 5 minutes. The resulting mixture was compressed at 217 mg / tablet using a tablet machine (FETTA Perfecta 1, Germany). The coating solution was prepared by using hypromellose, polyethylene glycol 6000, titanium dioxide, talcum powder, iron oxide yellow, and iron oxide red. Coating the above-prepared non-coated tablet with the above-prepared coating solution in a coating machine and then drying to obtain a tablet with a coating layer formed. The resulting tablets had a...
Embodiment 6 to 9
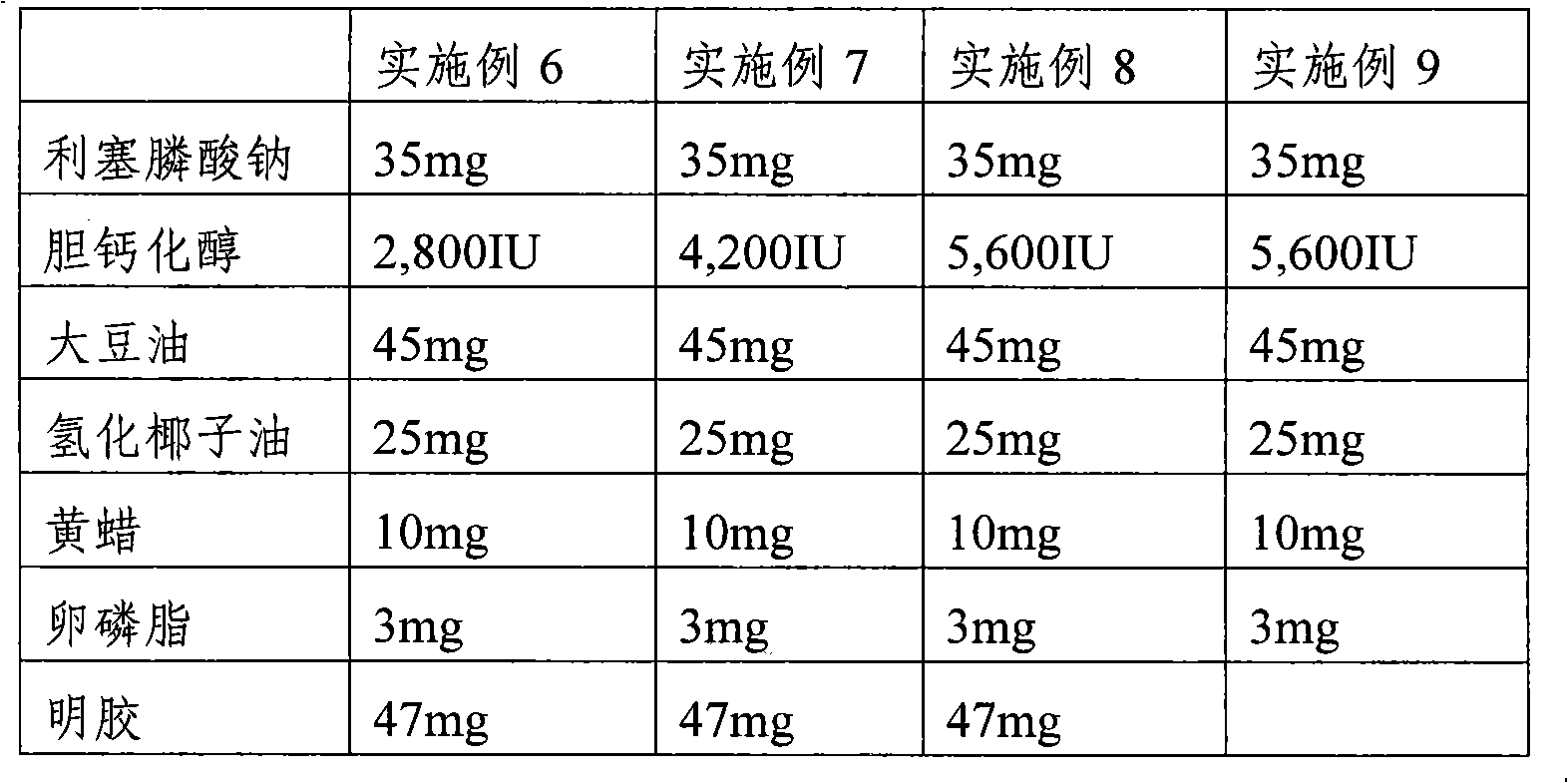
[0037] Soft capsules comprising risedronate sodium and cholecalciferol were prepared according to the ingredients and contents in Table 2 below.
[0038] Mix risedronate sodium (the content in Table 1 indicates the content as anhydrous), cholecalciferol, soybean oil, hydrogenated coconut oil, yellow wax, and lecithin, and then use a homogenizer (Homogenizer) to make the mixture uniform, A content solution was obtained. Gelatin or succinylated gelatin, mixed concentrated glycerin, 70% sorbitol solution, purified water, yellow No. 5, yellow No. 203, and titanium dioxide are prepared to obtain the soft capsule capsule material solution. In a continuous soft capsule filling machine, the above-prepared soft capsule material solution is used to fill the content solution obtained above, and dry to prepare soft capsules. The average amount of internal solution contained in each capsule of the prepared soft capsule is about 135 mg.
[0039] Table 2
[0040]
[0041]
experiment example 1
[0042] Experimental example 1 Disintegration test
[0043] Under the following conditions, disintegration experiments were carried out on the tablets and capsules prepared in Examples 1 to 9.
[0044] (1) Disintegration test conditions
[0045] Specimen: 6 tablets or 6 capsules
[0046] Disintegration fluid: water
[0047] Temperature: 37.0±0.5℃
[0048] (2) Disintegration test results
[0049] Disintegration test results in water: all the tablets of Examples 1 to 5 were disintegrated within 5 minutes, and all the soft capsules of Examples 6 to 9 were disintegrated within about 30 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com