Pharmaceutical compositions comprising bisphosphonate derivatives and high-dose cholecalciferol
A technology for cholecalciferol and composition, which is applied in the field of pharmaceutical compositions containing bisphosphonate derivatives and high-dose cholecalciferol, can solve the problems of difficulty in preparing calcitriol, inability to increase vitamin D, and the like, and achieves the Increase drug compliance, resolve unstable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-6 and comparative example 1-3
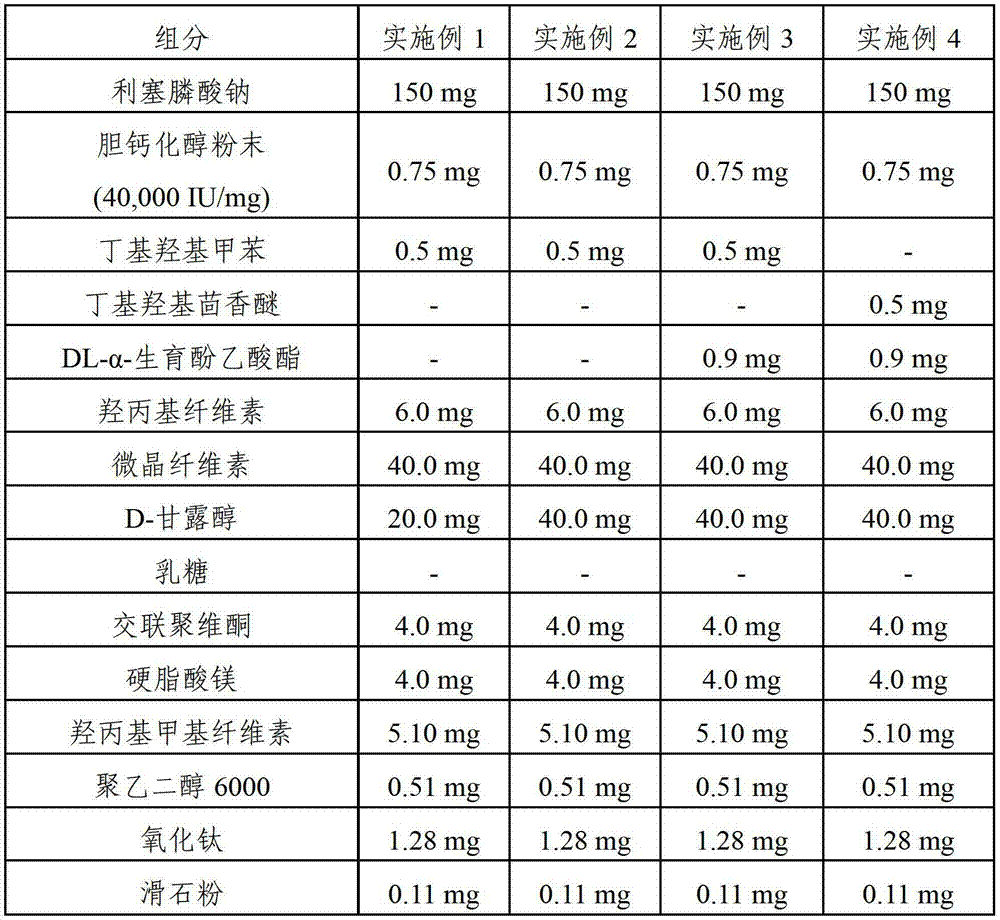
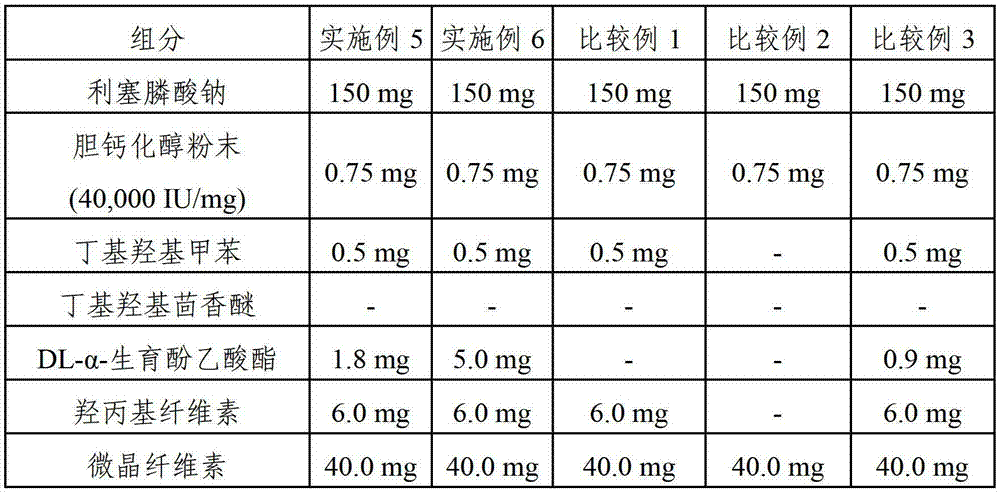
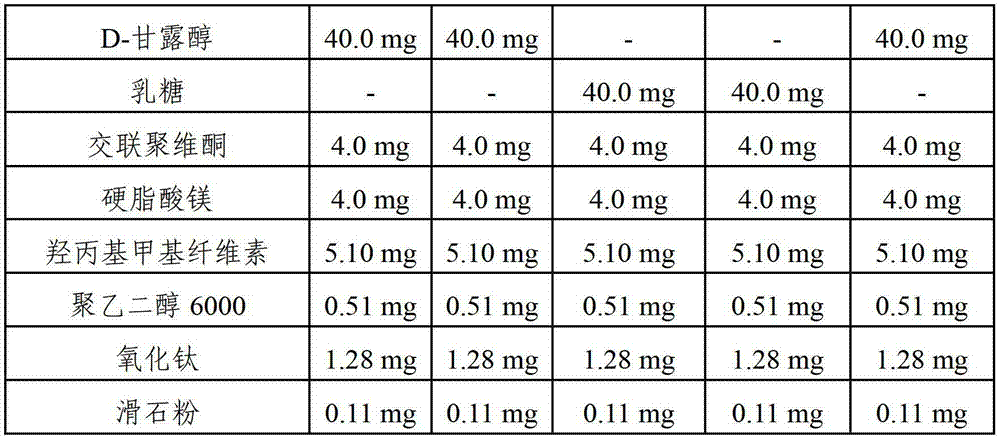
[0050] Examples 1-6 and Comparative Examples 1-3. Film-coated tablets containing risedronate sodium and cholecalciferol
[0051] Each film-coated tablet containing risedronate sodium and cholecalciferol was prepared according to the components and amounts shown in Tables 1 and 2 below. The amounts shown in Tables 1 and 2 are per tablet.
[0052] Mix cholecalciferol powder (40,000 IU / mg), first stabilizer (butylated hydroxytoluene, butylated hydroxyanisole and / or DL-α-tocopheryl acetate) and hydroxypropyl cellulose under stirring Dissolved in ethanol. The resulting solution was mixed with microcrystalline cellulose in a high speed mixer for about 30 seconds to obtain a slurry. The resulting slurry was evenly spread on a dry flat plate, then dried at a temperature of about 40° C., followed by grinding with a grinder (Fitz mill), thereby obtaining granules. Pass the granules through a 40-mesh sieve to obtain granules with cholecalciferol adsorbed on the surface of the microcry...
Embodiment 7-9
[0061] Examples 7-9. Film-coated tablets containing ibandronate sodium and cholecalciferol
[0062] Each film-coated tablet containing ibandronate sodium and cholecalciferol was prepared according to the components and amounts shown in Table 3 below. The amounts shown in Table 3 are per tablet.
[0063] Mix cholecalciferol powder (40,000 IU / mg), first stabilizer (butylated hydroxytoluene, butylated hydroxyanisole and / or DL-α-tocopheryl acetate) and hydroxypropyl cellulose under stirring Dissolved in ethanol. The resulting solution was mixed with microcrystalline cellulose in a high speed mixer for about 30 seconds to obtain a slurry. The resulting slurry was evenly spread on a dry flat plate, then dried at a temperature of about 40° C., followed by grinding with a grinder (Fitz mill), thereby obtaining granules. Pass the granules through a 40-mesh sieve to obtain granules with cholecalciferol adsorbed on the surface of the microcrystalline cellulose.
[0064] The granules ...
experiment example 1
[0069] Experimental Example 1: Stability Test
[0070] Stability tests were performed on the film-coated tablets prepared in Examples 1-9 and Comparative Examples 1-3. All the tablets were exposed at 50° C. for 2 weeks, and on the 7th and 14th days, the amount of active ingredients in the tablets, ie the amount of risedronate sodium or ibandronate sodium and cholecalciferol, was determined.
[0071] (1) The amount of risedronate sodium or ibandronate sodium
[0072] According to the stability test, the amount of risedronate sodium or ibandronate sodium determined on the 7th day and the 14th day is shown in Table 4.
[0073] Table 4
[0074]
[0075] Example 9
[0076] From the results shown in Table 4, it can be confirmed that risedronate sodium and ibandronate sodium maintained stability in the preparation of the present invention and in the preparation of the comparative example.
[0077] (2) The amount of cholecalciferol
[0078] According to the s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com