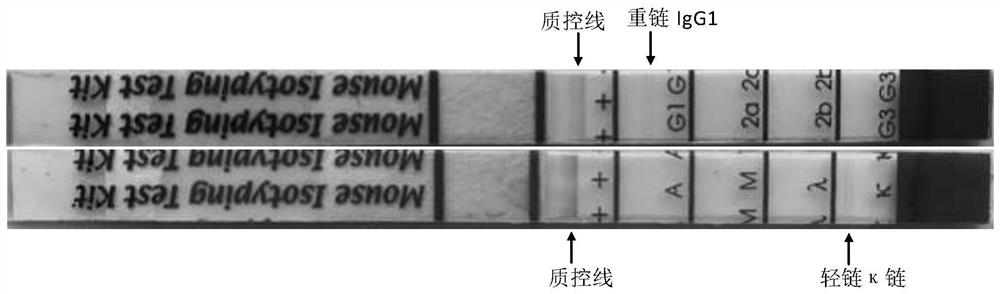
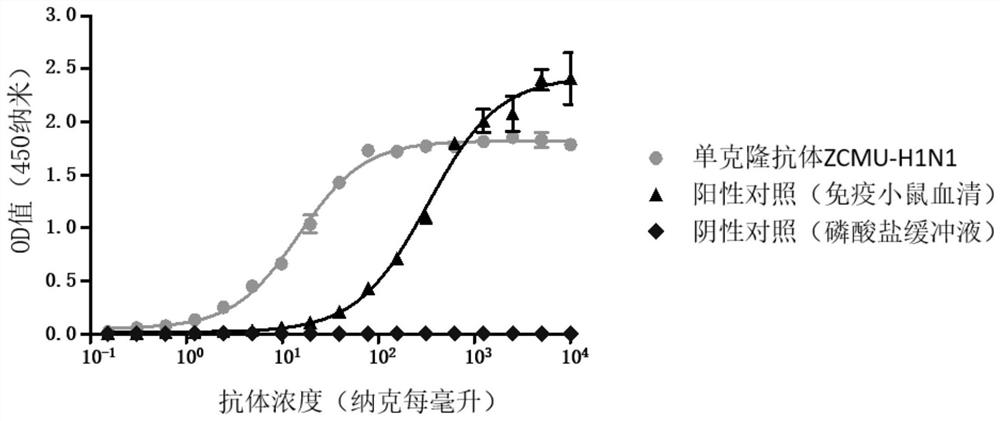
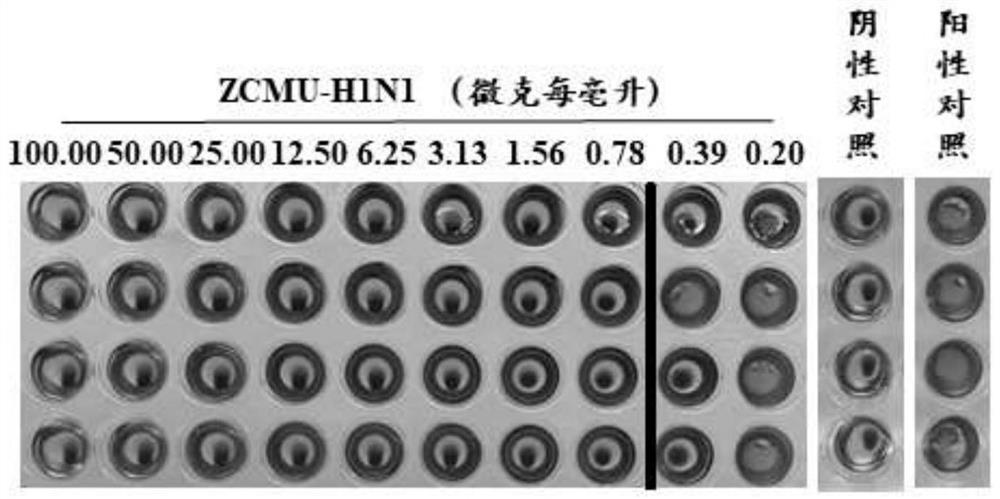
Anti-H1N1 influenza virus hemagglutinin protein monoclonal antibody ZCMU-H1N1 with neutralizing activity and application thereof
A ZCMU-H1N1, monoclonal antibody technology, applied in antiviral immunoglobulins, antiviral agents, antibodies, etc., can solve the problems of decreased antiviral treatment efficacy, oseltamivir resistance, and influenza virus resistance mutations.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1. Preparation method of monoclonal antibody against H1N1 influenza virus hemagglutinin protein
[0044] (1) Immunization of mice: For the first immunization, the H1N1 influenza virus hemagglutinin whole protein and adjuvant were mixed uniformly in equal volume, and the total volume was 600 microliters. Each BALB / C mouse 0.1 ml (containing 30 micrograms of H1N1 influenza virus hemagglutinin whole protein antigen) was intramuscularly injected into the inner thigh. On the 21st day, boost the immunization in the same way. On the 35th day, a small amount of tail blood was collected for enzyme-linked immunosorbent assay, and the antibody titer reached 1:100,000, followed by a booster immunization by tail vein injection, and cell fusion was performed 3 days later.
[0045] (2) Culture of mouse myeloma cell SP2 / 0: The SP2 / 0 myeloma cell line from BALB / C mice was cultured and passaged in DMEM medium containing 10% bovine serum, and incubated at 37°C saturated with 5% c...
Embodiment 2
[0055] Example 2. Antiviral effect of monoclonal antibody ZCMU-H1N1 against HA protein of H1N1 influenza virus
[0056] (1) Microneutralization experiment: ①H1N1 influenza virus (A / Michigan / 45 / 2015) was titrated by half of the tissue cells infective dose; ②MDCK cells were inoculated in 96-well culture plates, 2×10 per well 4 Cells were cultured in a 37°C incubator saturated with 5% carbon dioxide for 24 hours; ③ The virus was diluted with 0.2% trypsin-containing virus culture medium to 100 times the infection dose of half the tissue cells per 50 microliters; ④ In a 96-well culture plate 10 micrograms per milliliter of the monoclonal antibody ZCMU-H1N1 were diluted to different concentrations (1:1, 1:2, 1:4, 1:8, 1:16, 1:32, 1:16, 1:32, and 64, 1:128, 1:256, 1:521), 50 microliters per well; ⑤ Add 50 microliters of 100-fold half histiocytic infection dose per 50 microliters of virus solution to the wells with antibodies, and mix For each dilution, 4 replicate wells were made; t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com