Capped composition as well as preparation method and in-vitro transcription reaction system thereof
An in vitro transcription and reaction system technology, applied in the fields of chemical and biological engineering, can solve the problems of large steric hindrance, low capping efficiency, poor capping enzyme recognition efficiency, etc., to achieve improved capping efficiency, increased yield, and biocompatibility excellent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
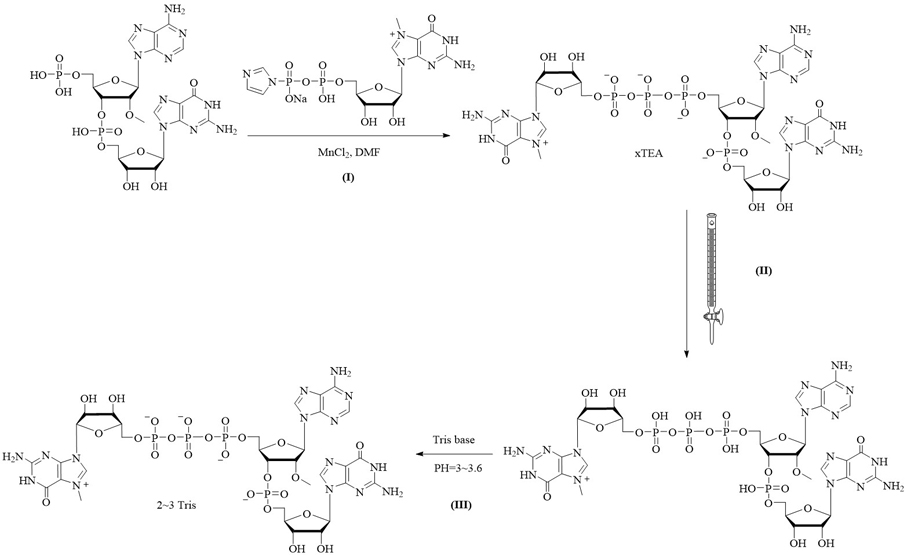
[0058] A capping composition combining figure 1 , prepared by the following method:
[0059] I: Compound m7GDP imidazolium salt (2mmol) was dissolved in MnCl containing 2 (2 mmol) in DMF and added to a 5'-phosphorylated dinucleotide (1 mmol; triethylammonium salt) in DMF. The reaction was stirred at room temperature. After 24 hours, the reaction was quenched with 10 mL of 0.25 M EDTA solution. The mixture was loaded onto a DEAESephadex column (3 x 50 cm). The product was eluted using a linear gradient of 0-1.0M TEAB eluent. The eluted product with HPLC purity >97% was collected and concentrated to give the capped analog as triethylamine salt form (c) at a concentration of 120 mM;
[0060] II: the product of converting the triethylamine salt form of the capped analog to the free acid form of the same concentration by a cation exchange resin;
[0061] III: dropwise add 0.2 M TRIS base to the product solution to PH=3~3.6, then concentrate and constant volume to obtain the f...
Embodiment 2
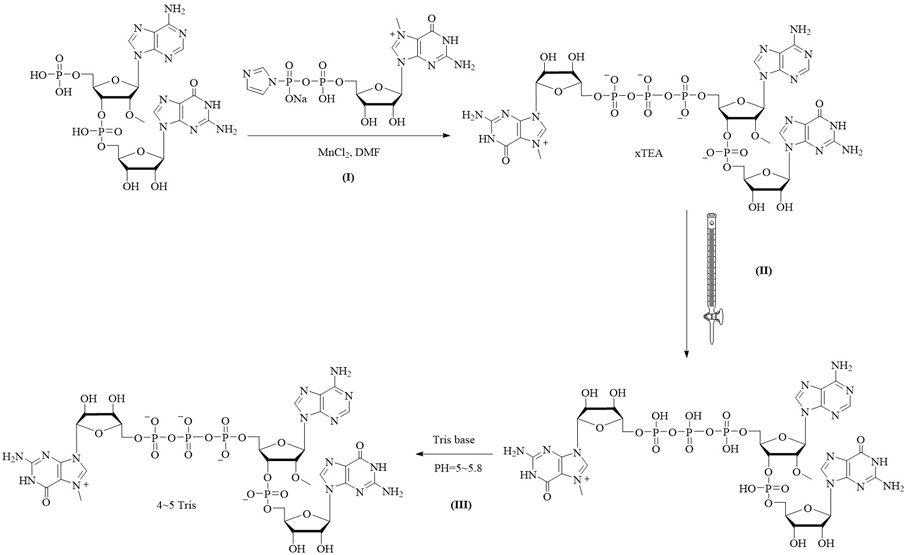
[0063] A capping composition combining figure 2 , prepared by the following method:
[0064] I: Compound m7GDP imidazolium salt (2 mmol) was dissolved in MnCl containing 2 (2 mmol) in DMF and added to a 5'-phosphorylated dinucleotide (1 mmol; triethylammonium salt) in DMF. The reaction was stirred at room temperature. After 24 hours, the reaction was quenched with 10 mL of 0.25 M EDTA solution. The mixture was loaded onto a DEAESephadex column (3 x 50 cm). The product was eluted using a linear gradient of 0-1.0M TEAB eluent. The eluted product with HPLC purity >97% was collected and concentrated to give the capped analog in the form of a triethylamine salt at a concentration of 120 mM;
[0065] II: converting the triethylamine salt form of the capped analog to a product of the same concentration as the free acid form by a cation exchange resin;
[0066] III: 0.2M TRIS base is added dropwise to the product solution to PH=5~5.8, and the final product is obtained by concen...
Embodiment 3
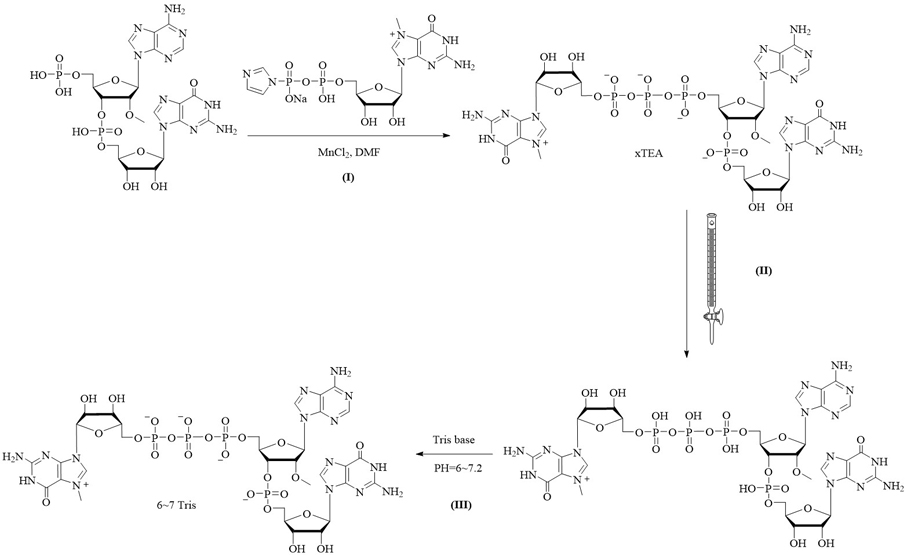
[0068] A capping composition combining image 3 , prepared by the following method:
[0069] I: Compound m7GDP imidazolium salt (2 mmol) was dissolved in MnCl containing 2 (2 mmol) in DMF and added to a 5'-phosphorylated dinucleotide (1 mmol; triethylammonium salt) in DMF. The reaction was stirred at room temperature. After 24 hours, the reaction was quenched with 10 mL of 0.25 M EDTA solution. The mixture was loaded onto a DEAESephadex column (3 x 50 cm). The product was eluted using a linear gradient of 0-1.0M TEAB eluent. The eluted product with HPLC purity >97% was collected and concentrated to give the capped analog in the form of a triethylamine salt at a concentration of 120 mM;
[0070] II: converting the triethylamine salt form (c) of the capped analog to the product of the free acid form at the same concentration by a cation exchange resin;
[0071] III: 0.2M TRIS base is added dropwise to the product solution to PH=6~7.2, and the final product is obtained by c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com