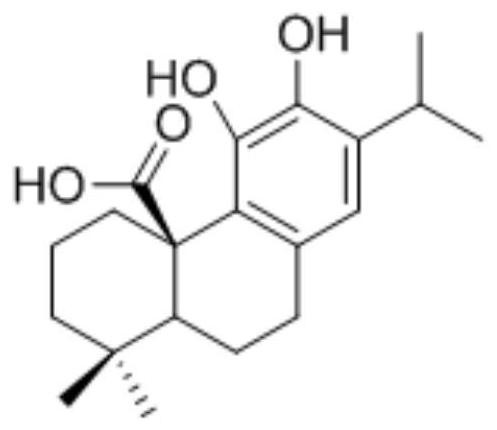
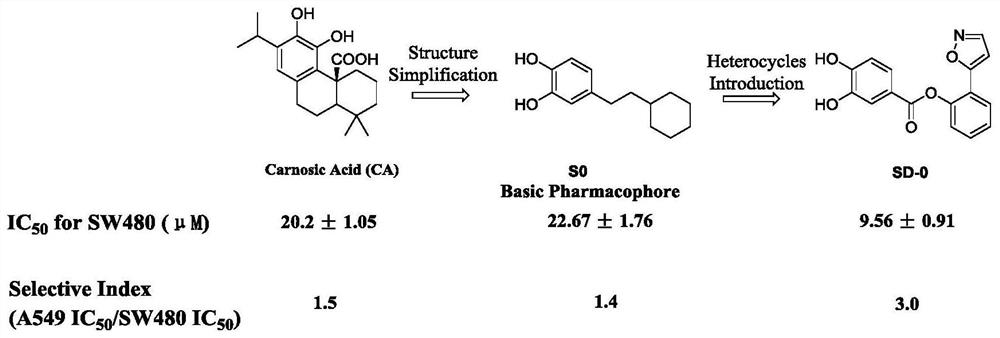
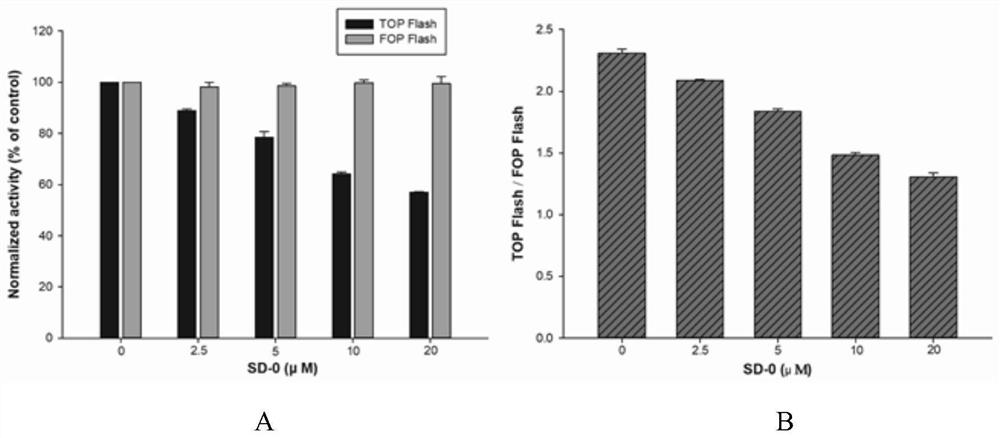
2-(isoxazole-5-yl) phenyl-3, 4-dihydroxy-benzoate and derivative thereof, synthetic method and application of 2-(isoxazole-5-yl) phenyl-3, 4-dihydroxy-benzoate and derivative thereof
A technology of hydroxybenzoate and dimethoxybenzoate is applied in the fields of 2-phenyl-3,4-dihydroxybenzoate and its derivatives and synthesis to inhibit cell proliferation and reaction Mild conditions and easy post-processing results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] Compound 1: Preparation of 2-bromo-6-(isoxazol-5-yl)phenyl-3,4-dihydroxybenzoate:
[0127] (1) Synthesis of 2-bromo-6-(5-isoxazolyl) phenol:
[0128] 1-(3-Bromo-2-hydroxyphenyl)ethanone (5 mmol) and DMF-DMA (7.5 mmol) were added to a 50 mL flask, an appropriate amount of Toluene was added to dissolve, the reaction mixture was heated to 90 °C, and stirred at 90 °C for 2 h , then cooled to room temperature, Ethanol (5 mL) and NH were added to the reaction mixture 2 OH.HCl (7.6 mmol), then the solution was heated to 78 °C and stirred for 1 h, and the compound 2-bromo-6-(5-isoxazolyl)phenol was extracted and isolated.
[0129] NMR spectral data: 1 H NMR (600MHz, MeOD): δ 8.44 (d, J=1.9Hz, 1H), 7.84 (dd, J=7.9, 1.6Hz, 1H), 7.59 (dd, J=7.9, 1.6Hz, 1H), 6.96(d,J=1.9Hz,1H),6.92(t,J=7.9Hz,1H).
[0130] (2) Synthesis of 2-bromo-6-(isoxazol-5-yl)phenyl-3,4-dimethoxybenzoate:
[0131] 3,4-Dimethoxybenzoic acid (5.2 mmol) was added to a 50 mL flask, dissolved with an appropriat...
Embodiment 2
[0137] Compound 2: Preparation of 2-(3-methylisoxazol-5-yl)phenyl-3,4-dihydroxybenzoate:
[0138] (1) Synthesis of 2-(3-methylisoxazol-5-yl)phenol:
[0139] 2'-hydroxyacetophenone (5 mmol), pyridine 7.5 mmol), acetic anhydride (7.5 mmol) were added to a 50 mL flask, an appropriate amount of DCM was added to dissolve, the reaction mixture was stirred at room temperature for 6 h, and the dichloromethane in the reaction system was spin-dried , pyridine (3 mL), DBU (10 mmol) were added, the reaction system was refluxed at 100 °C for 7 h, then cooled to room temperature, NH2OH.HCl (7.6 mmol) was added to the reaction mixture, then the solution was heated to 78 °C and stirred for 1 h, extracted The compound 2-(3-methylisoxazol-5-yl)phenol was isolated.
[0140] NMR spectral data: 1 H NMR (600MHz, MeOD): δ 7.78 (dd, J=7.8, 1.6Hz, 1H), 7.26 (ddd, J=8.3, 7.3, 1.7Hz, 1H), 6.97–6.91 (m, 2H), 6.77 (s,1H),2.32(s,3H).
[0141](2) Synthesis of 2-(3-methylisoxazol-5-yl)phenyl-3,4-dimethox...
Embodiment 3
[0147] Compound 3: Synthesis of 2-chloro-6-(isoxazol-5-yl)phenyl-3,4-dihydroxybenzoate:
[0148] According to the preparation method of compound 1, 1-(3-chloro-2-hydroxyphenyl)ethyl-1-one was used to replace 1-(3-bromo-2-hydroxyphenyl)ethanone, and other operations were the same. The product is a white solid.
[0149] NMR spectral data: 1 H NMR (600MHz, MeOD): δ8.37 (d, J=1.9Hz, 1H), 7.92 (dd, J=8.0, 1.5Hz, 1H), 7.66 (ddd, J=8.1, 4.3, 1.8Hz, 2H) ), 7.61(d, J=2.1Hz, 1H), 7.44(t, J=8.0Hz, 1H), 6.93(d, J=8.3Hz, 1H), 6.63(d, J=1.9Hz, 1H). 13 C NMR (151MHz, MeOD): δ165.5, 164.9, 153.2, 152.2, 146.7, 145.7, 132.9, 130.3, 128.7, 127.8, 124.9, 124.5, 120.5, 118.0, 116.4, 103.7
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com