Novel ribonucleic acid and pharmaceutical composition based on same
A base and base sequence technology, applied in the field of novel ribonucleic acid, can solve the problems of low stability of RNaseT1 and the length of dsRNA analogs are not constant, and achieve the purpose of reducing antigenic mass, improving polarization cross-protection reaction, and enhancing innate immunity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0213] In vivo dendritic cell (DC) activation by hsRNA-based next adjuvant
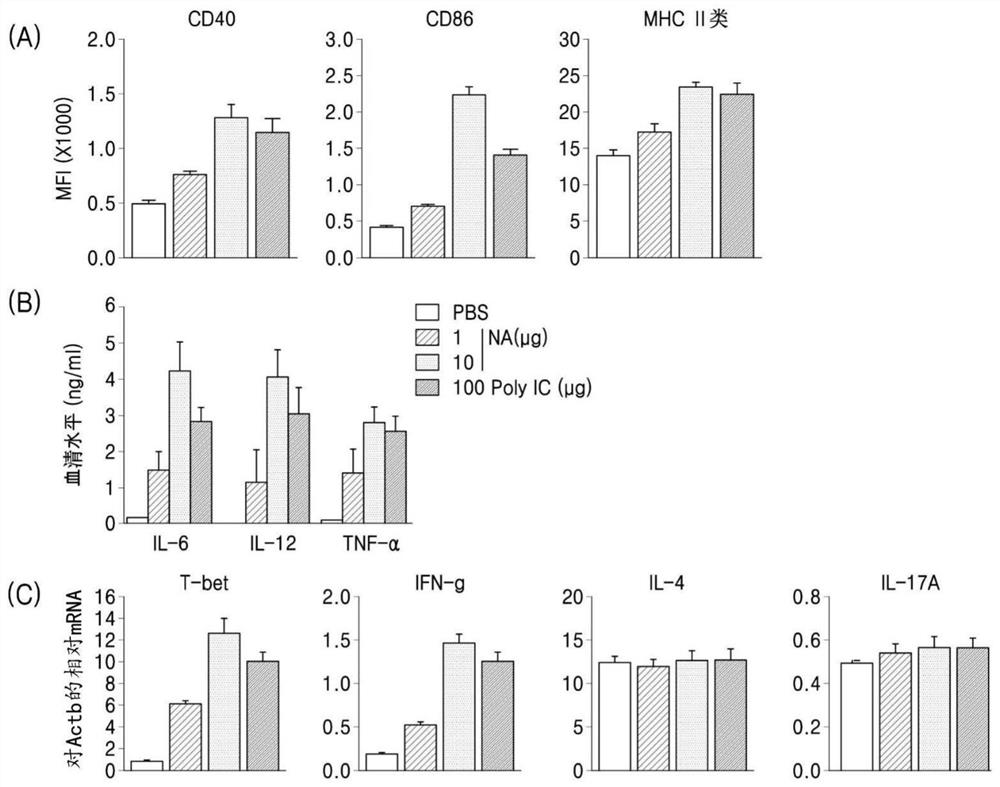
[0214] This example demonstrates in vivo innate immune activity by hsRNA-based next adjuvant (abbreviated as NA) by intraperitoneal (i.p: intraperitoneal) injection into C57BL / 6 mice ( figure 1 ).
[0215] An example of the NA (alias NVT or VP10) of the invention (Table 2) induces differentiation of dendritic cell markers (CD40, CD86 and MHC-II) to levels equal to or higher than 100 μg of Poly(I:C) ( figure 1 ). NA further induces type 1 interferon beta (IFN-β), which correlates with the skewness of naive CD4 T cells to Th1 CD4 T cells and the secretion of IL-6 and IL-12 ( figure 1 ). In addition, NA induced T-bet (a Th1 cell marker) and IFN-γ, but not Th2 cytokines (IL-4) and Th17 cytokines (IL-17A) ( figure 1 ). NA strongly activated DCs compared to the Poly(I:C) positive control group.
Embodiment 2
[0217] In vivo DC activation depends on hsRNA length rather than sequence
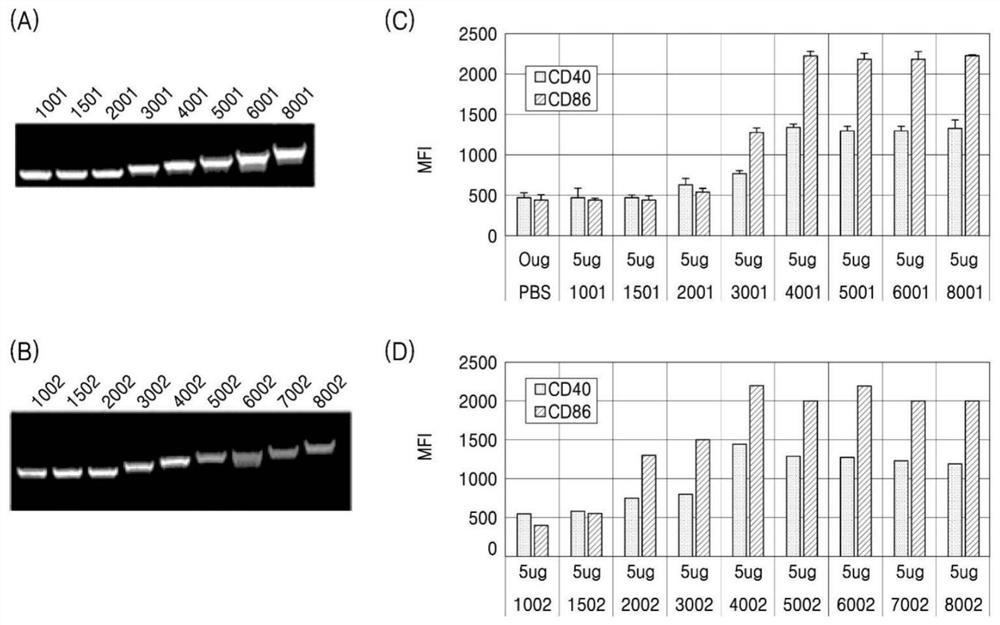
[0218] hsRNA was prepared from two independent backbones of different lengths, where the dsRNA region varied from 106 to 806 bp, but the ssRNA overhang at both ends in this Example 2 was constant at 17 bases ( figure 2 A and figure 2 B), and splenic DC activation was tested intraperitoneally in mice.
[0219] The level of DC activation increases with hsRNA length, as assessed by induction of CD40 and CD86, showing high activity at 400 to 900 bases ( figure 2 C and figure 2 D).
[0220] All hsRNAs showed better activity than the positive control Poly(I:C).
[0221] Because all ssRNA overhangs are non-complementary, with 17 bases at each end, for a total of 34 bases, differences in DC activation from hsRNAs must arise with increasing dsRNA length. Therefore, dsRNAs with a range of about 406 to 806 bp showed high activity.
[0222] When rechecking the 540hsRNA (506bp dsRNA), which showed high ac...
Embodiment 3
[0224] Antigen-specific antibody responses depend on hsRNA length
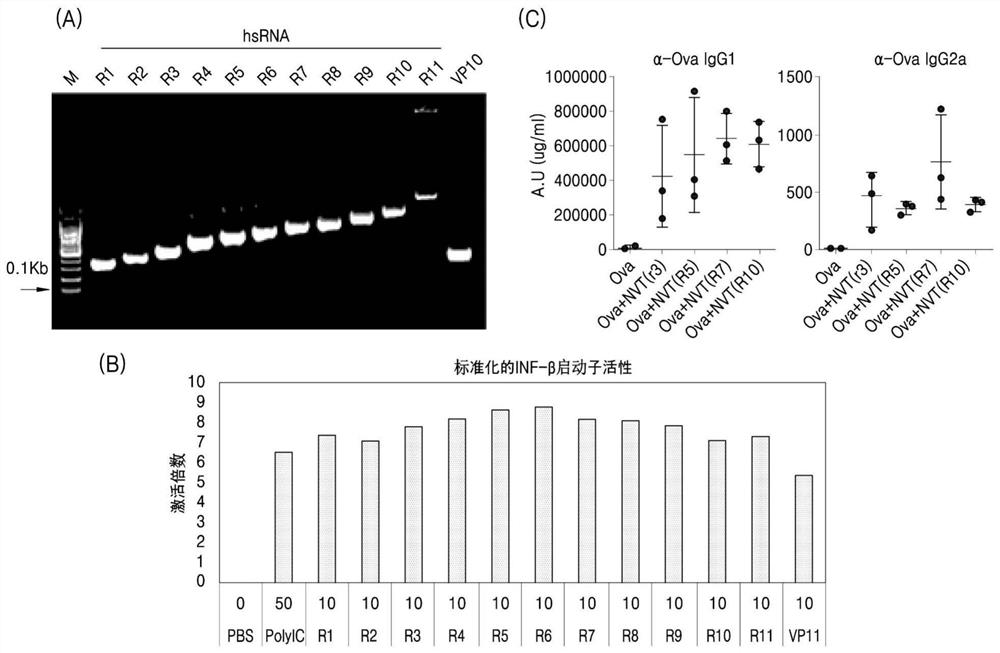
[0225] More hsRNAs with a broad range (353 to 1682 bases) were derived from a third backbone vector, different from the first two vectors shown in Example 2 ( image 3 A). After transient co-transfection of these RNAs with an IFN-β promoter-driven vector into HEK 293 cells, a surrogate reporter for innate immune activation was identified ( image 3 B). IFN-β promoter activity increased proportionally to the length of hsRNA and dsRNA, reaching a maximum in the range of 406 bp to 806 bp. Notably, this activity was independent of the specific RNA sequence, which is consistent with Example 2.
[0226] Some of these (R3, R5, R7 and R10) were complexed with the model antigen OVA and tested in vivo for their induction of anti-OVA IgG in mice.
[0227] Consistent with the above, high levels of anti-OVA IgG1 and Th1-mediated anti-OVA IgG2 ( image 3 C).
[0228] Taken together, hsRNA (140 to 1682 bp) and dsRNA (1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com