Preparation method of 6-chloro-2-methyl-2H-indazole-5-amine
A technology of methyl and indazole is applied in the field of preparation of 6-chloro-2-methyl-2H-indazol-5-amine, and achieves the effects of low price, high operational safety and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
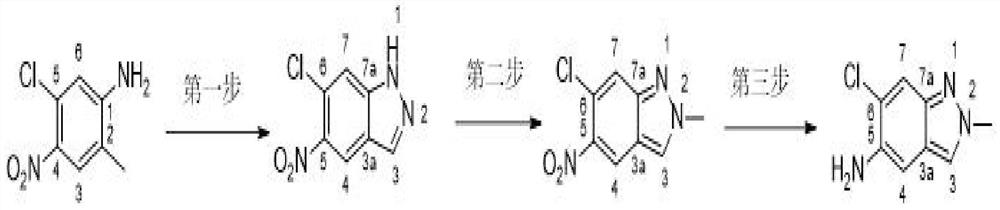
[0022] like figure 1 Shown a kind of preparation method of 6-chloro-2-methyl-2H-indazol-5-amine, comprises the steps:
[0023] Step 1: Add 2-methyl-4-nitro-5-chloroaniline to the solvent, add diazotization reagent under acidic conditions, and obtain 6-chloro-5-nitro- 1H-indazole;
[0024] Step 2: Add the 6-chloro-5-nitro-1H-indazole in step 1 to the solvent, and add a methylating reagent to obtain 6-chloro-2-methyl-5-nitrogen, a methyl product at position 2 yl-2H-indazole;
[0025] Step 3: Add the 2-position methyl product 6-chloro-2-methyl-5-nitro-2H-indazole in step 2 into the solvent and add a reducing agent to obtain 6-chloro- 2-Methyl-2H-indazol-5-amine.
Embodiment 1
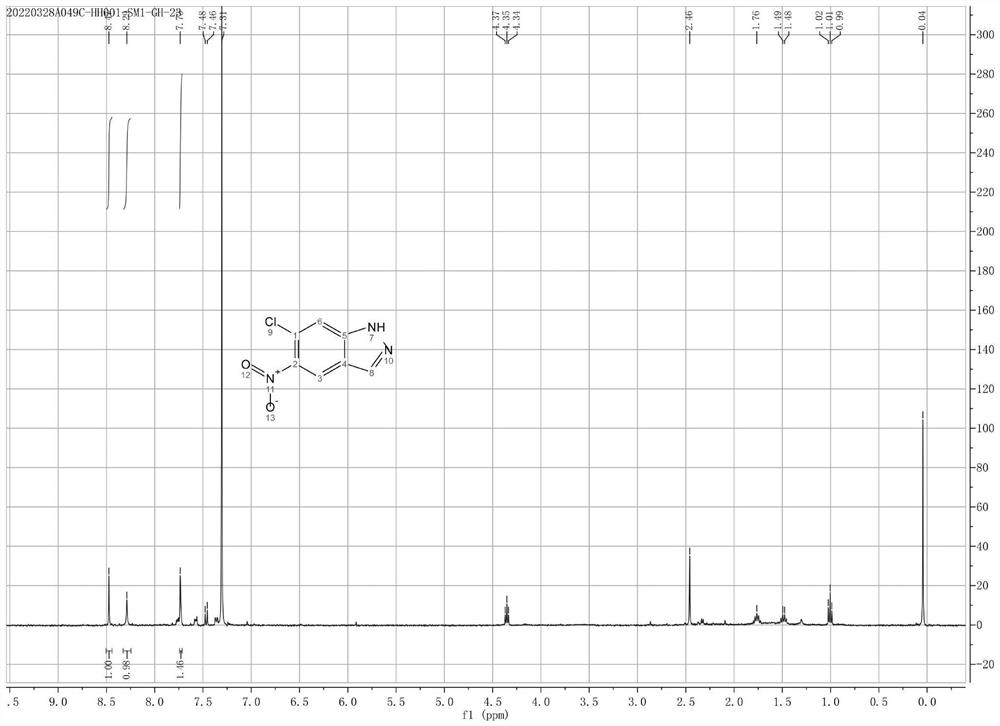
[0027] In a 100 mL three-necked flask with stirring and a thermometer, add 1.87 g of 2-methyl-4-nitro-5-chloroaniline, 50 mL of acetic acid and 10 mL of water, stir, and cool to 0°C. Dissolve 1.04 g of sodium nitrite in 10 mL of water and add it to the reaction system. After dripping, the reaction was continued at 10° C. for 6 hours, and the temperature was naturally raised to room temperature to react overnight, and monitored by TLC (EA / PE=1:3). The reaction system was concentrated to remove acetic acid. Saturated aqueous sodium bicarbonate solution and ethyl acetate were added to separate the layers; the aqueous phase was extracted twice with ethyl acetate, and the organic phases were combined. Dry over anhydrous sodium sulfate and concentrate. 1.2 g of 6-chloro-5-nitro-1H-indazole (B) was obtained, yield: 61%. 1H NMR (400 MHz, CDCl3) δ 8.47 (s, 1H), 8.29 (s, 1H), 7.79 (s, 1H).
Embodiment 2
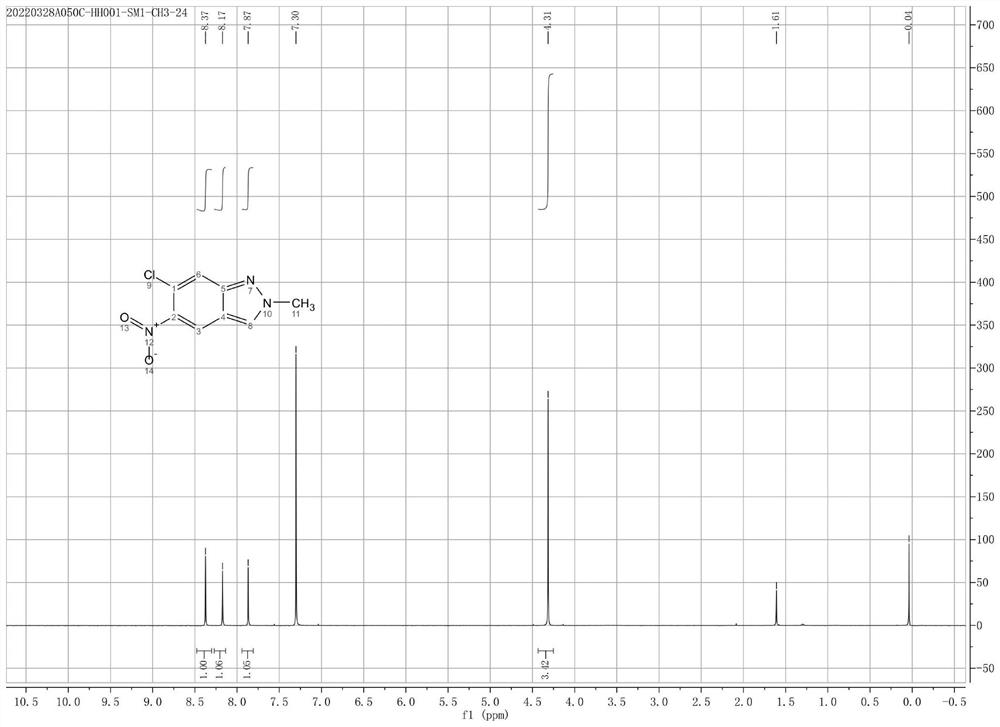
[0029] In a 500 mL three-necked flask with stirring and a thermometer, add 1.97 g of 6-chloro-5-nitro-1H-indazole and 200 mL of ethyl acetate, then add 1.92 g of trimethyloxonium tetrafluoroboric acid, and stir at room temperature. TLC (EA / PE=1:1) monitoring, the disappearance of starting materials stopped the reaction. The reaction system was concentrated to remove ethyl acetate. Column chromatography obtained 1.85g of 6-chloro-2-methyl-5-nitro-2H-indazole, yield 88%: 1HNMR (400MHz, CDCl3) δ8.37(s,1H), 8.17(s,1H) ), 7.87(s, 1H), 4.31(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com