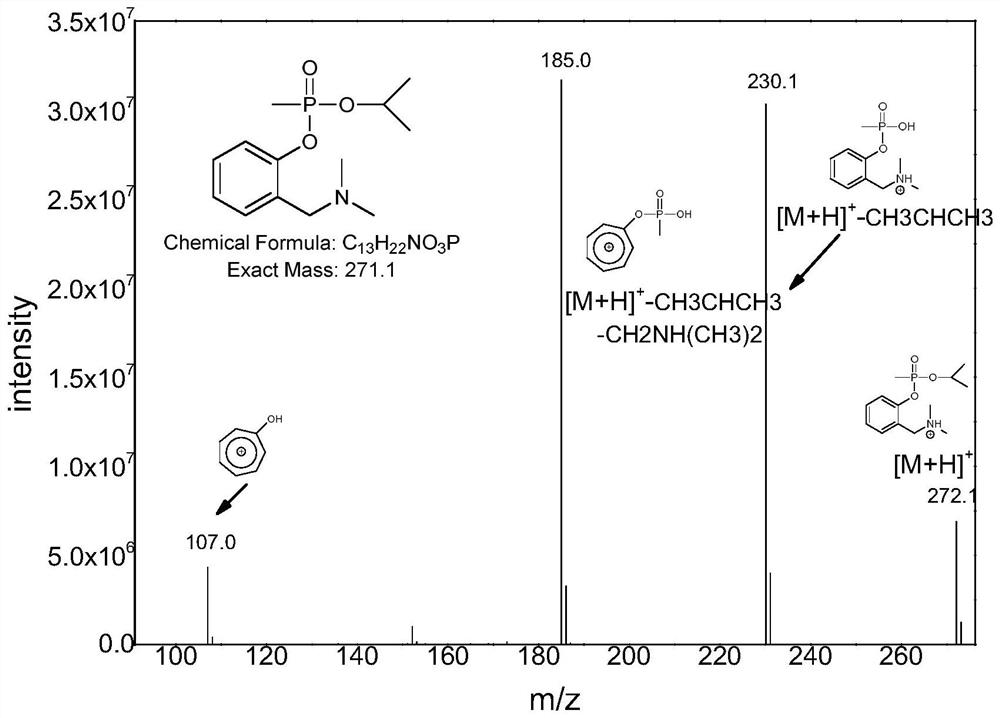
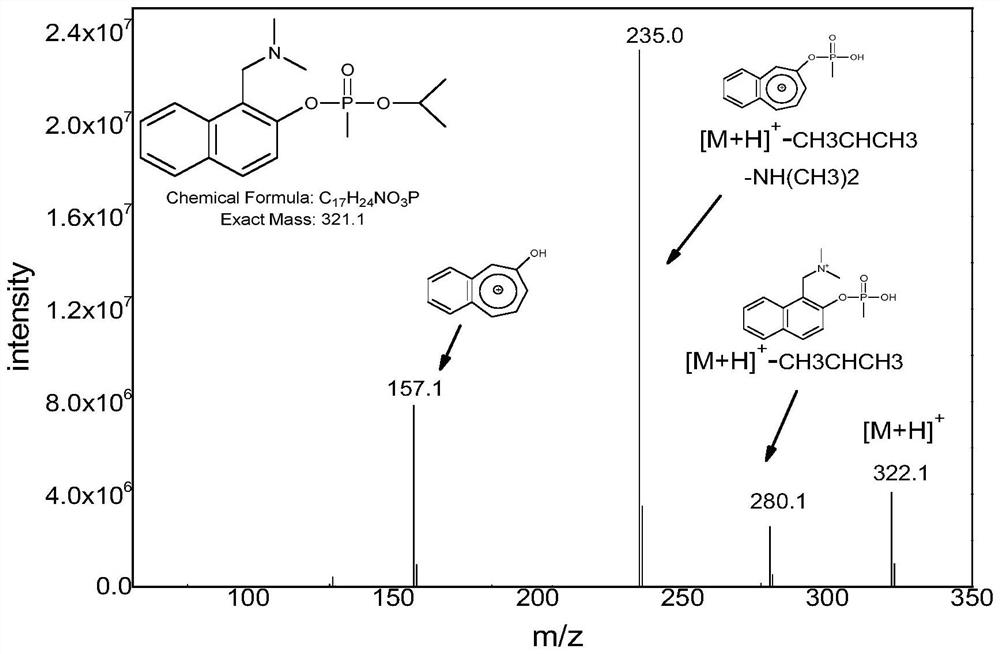
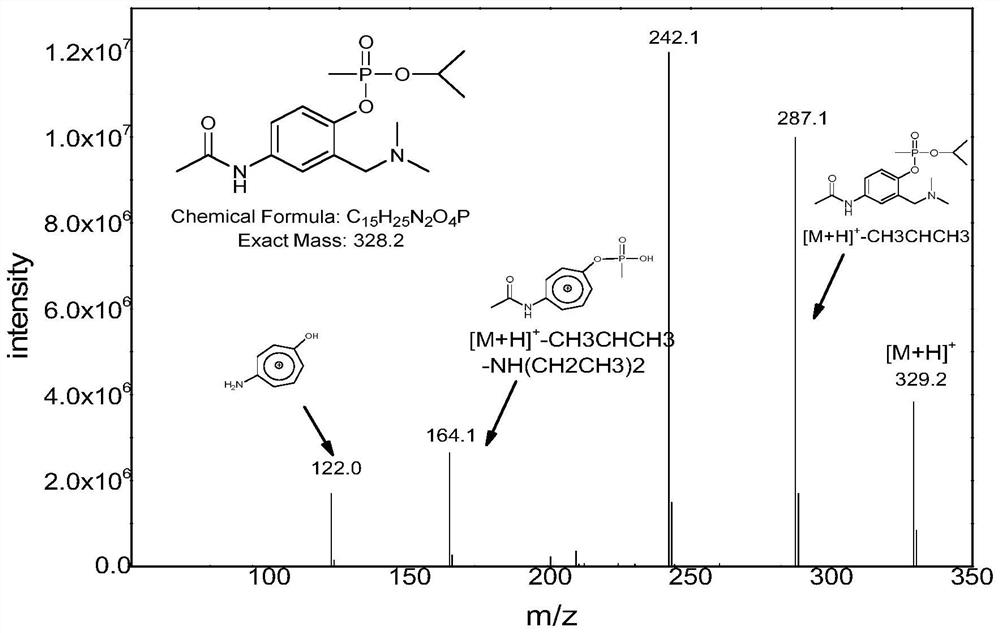
Derivatization reagent, kit and method for detecting nerve agent
A neurological, kit-based technology, applied in the field of analytical chemistry, can solve the problems of weak characteristic signal, high detection limit, and difficulty in accurately measuring the content of toxic agents.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] Compounds of formula I
[0019] The compound shown in the formula I of the present invention can be used as the derivatization reagent of the nerve agent to detect the nerve agent in the sample, and its structural formula is as follows:
[0020]
[0021] where R 1 and R 2 independently C 1-4 Alkyl, or R 1 and R 2 The N atoms to which they are attached form nitrogen-containing heterocycles or nitrogen-containing heteroaromatic rings.
[0022] In certain embodiments, R 1 and R 2 independently C 1-4 Alkyl, or R 1 and R 2 The N atoms to which they are attached form five- or six-membered nitrogen-containing heterocycles or nitrogen-containing heteroaromatic rings.
[0023] In certain embodiments, R 1 and R 2 Each is independently methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl or tert-butyl.
[0024] In certain embodiments, R 1 and R 2 Each is independently methyl, ethyl or n-propyl.
[0025] In certain embodiments, R 1 and R 2 Each is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com