Process for preparing monomethyl fumarate
A technology of monomethyl fumarate and monomethyl maleate, which is applied in the technical field of preparing monomethyl fumarate to achieve good environmental performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
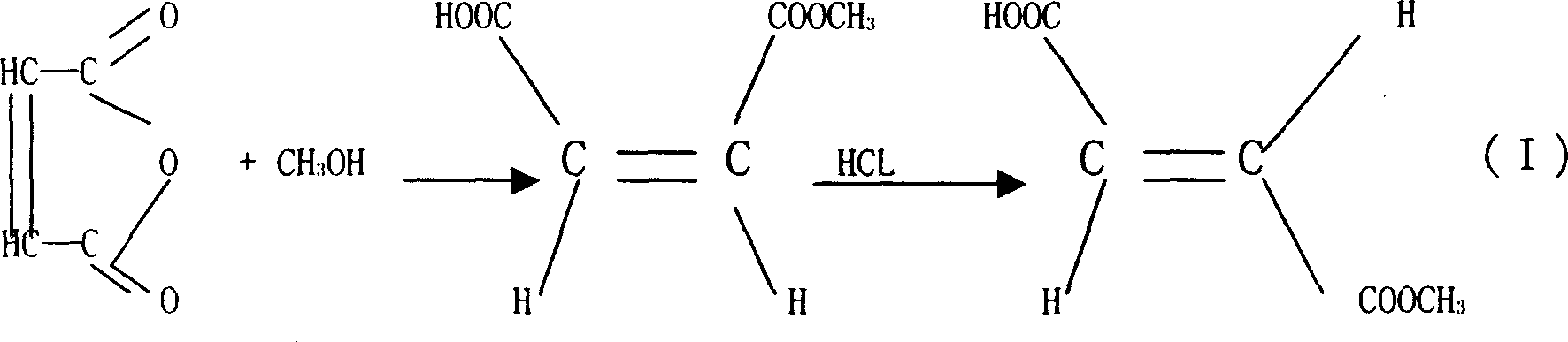
[0018] Embodiment 1: Maleic anhydride and methyl alcohol are dropped in the reaction tank by the ratio (weight ratio) of 98: 32, keep stirring with 45 rev / mins, and reaction system begins to drop in temperature, and this is because maleic anhydride is soluble in methanol Due to physical heat absorption. However, when part of the maleic anhydride dissolves, it will immediately undergo esterification reaction with methanol, and the temperature of the reaction system begins to rise. When the temperature rises to 50°C, cool the reaction tank with cooling water to keep the reaction temperature at 30--70°C. After 3 hours, it was cooled to room temperature to obtain monomethyl maleate.
Embodiment 2
[0019] Embodiment 2: the monomethyl maleate obtained by reaction is placed in the isomerization container, the concentrated hydrochloric acid of 0.5% of the monomethyl maleate weight is added, mix well, be placed in the container that can carry out air-cooling or water-cooling , keep the temperature of the whole isomerization reaction system at 60°C. After standing still for 50 hours, the monomethyl fumarate was taken out, and the powdery monomethyl fumarate product was obtained after crushing, with a yield of not less than 95%.
Embodiment 3
[0020] Embodiment 3: Solvent isomerization: place reaction gained monomethyl maleate in reaction pot, add ethyl acetate, the weight ratio of monomethyl maleate and ethyl acetate is 1: 3, then add maleic acid 1% concentrated hydrochloric acid of monomethyl ester weight, keep stirring at 50 rev / min, finish after 6 hours. During the isomerization process, cooling water is used to control the isomerization reaction temperature at about 50°C. Most of the monomethyl fumarate precipitates out in the form of precipitation, and it is centrifuged (3000 rpm) for 7 minutes to obtain monomethyl fumarate, which contains about 5-8% ethyl acetate, and placed at 50°C In the left and right ovens, the pure product of monomethyl fumarate was obtained after vacuum drying for 1 hour. This method is more complicated than the direct isomerization method. But because solvent exists in the isomerization process, the reaction temperature is easy to control, the product purity is higher, and the solvent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com