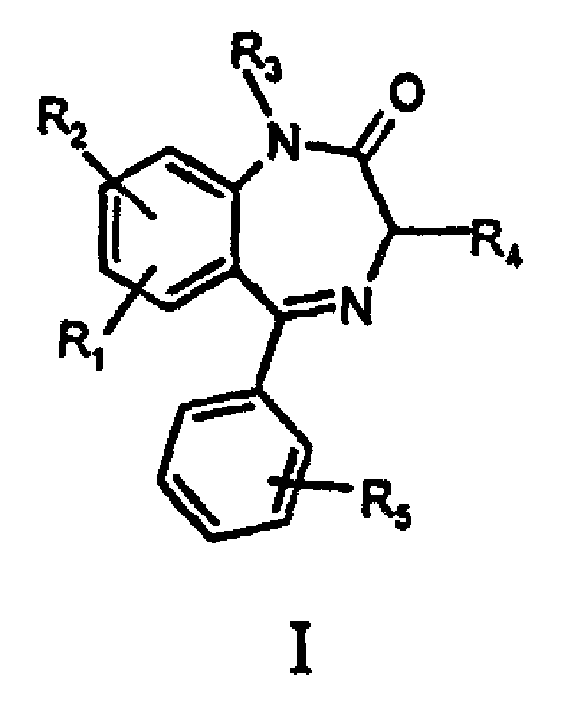
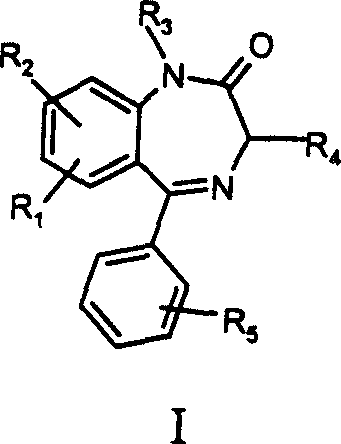
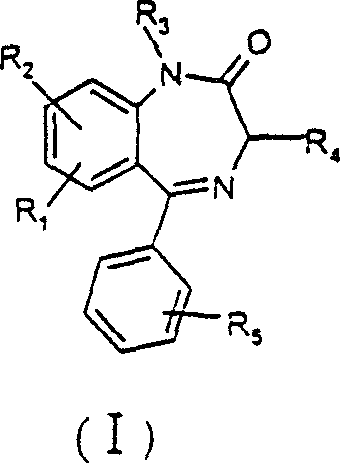
Benzodiazapine compounds containing imidazole propanamido and its preparing process and application
A technology containing imidazole propionamide group and imidazole propionamide group, which is applied in the fields of benzodiazepine compounds, transferase activity, and farnesyl protein transferase inhibitors, and can solve the problem of poor selectivity of enzymes and cells, Compound activity is not high, to achieve the effect of inhibiting the growth of cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1 7-nitro-1-ethoxycarbonylmethylene-5-phenylbenzodiazepine preparation of
[0066] In the 50ml round-bottomed flask, add 7-Xoctyl-5-phenylbenzodiazepine (2.0g 7.2mmol), potassium carbonate (20g 14.4mmol), 20ml acetonitrile and ethyl bromoacetate (1.8g 10.6mmol), React at room temperature for 2 hours. After the reaction, 50ml of water was added, extracted with ethyl acetate, concentrated under reduced pressure to remove ethyl acetate, and the residue was recrystallized with absolute ethanol to obtain 1.8g of white crystals. The crystal was determined to be 7-nitro-1-ethoxycarbonylmethylene-5-phenylbenzodiazepine. mp: 141-143°C, yield 81.6%.
Embodiment 2
[0067] Example 2 7-nitro-1-(2-methoxycarbonylethyl)-5-phenylbenzodiazepine preparation of
[0068] Proceed in the same manner as in Example 1, except that 1.8 g of methyl 3-bromopropionate is used instead of ethyl bromoacetate to obtain an oil. The oil, 7-nitro-1-(2-methoxycarbonylethyl)-5-phenylbenzodiazepine, was used in Example 5 without further purification.
Embodiment 3
[0069] Example 3 7-nitro-1-(3-ethoxycarbonylpropyl)-5-phenylbenzodiazepine preparation of
[0070] Proceed in the same manner as in Example 1, except that 1.8 g of ethyl 4-bromobutyrate is used instead of ethyl bromoacetate to obtain an oily substance. The oil, 7-nitro-1-(3-ethoxycarbonylpropyl)-5-phenylbenzodiazepine, was used in Example 6 without further purification.
[0071] 7-Amino-1-(ω-alkoxycarbonylalkyl)-5-phenylbenzodiazepine preparation of
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com