Lipophilic microparticles containing protein drug or antigen and formulation comprising same
A lipophilic, protein-based technology, applied in the field of lipophilic substance-coated particles, can solve problems such as the instability of liposome particle structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
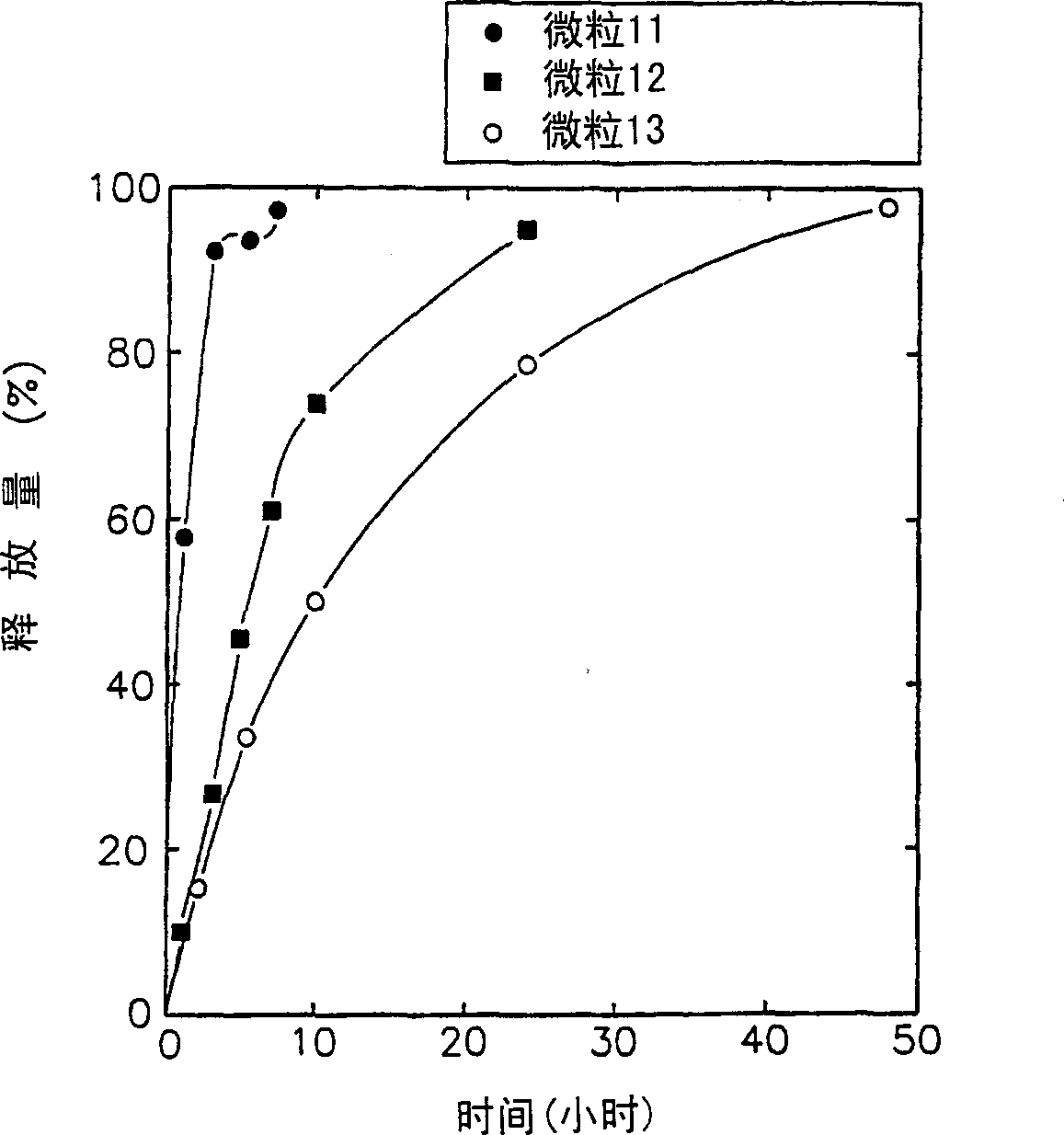
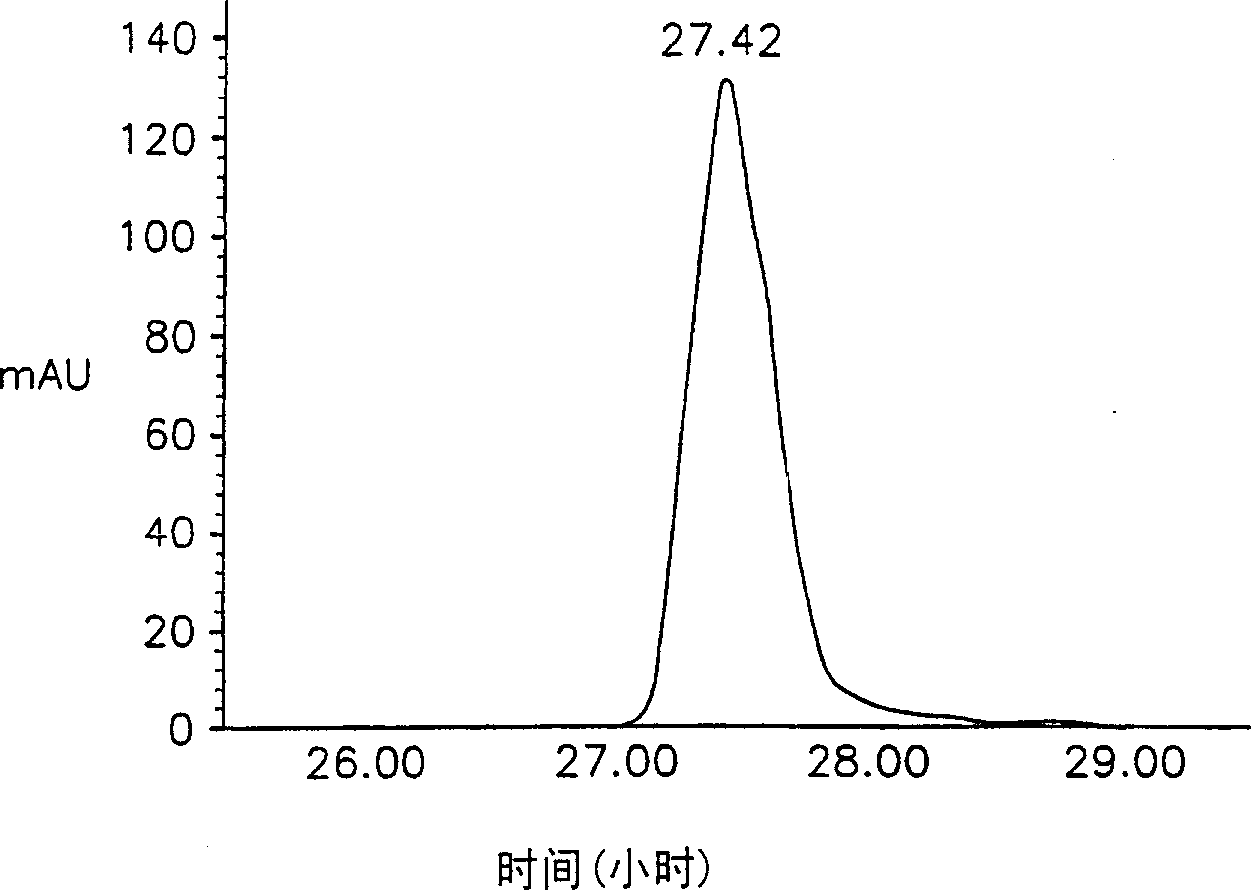
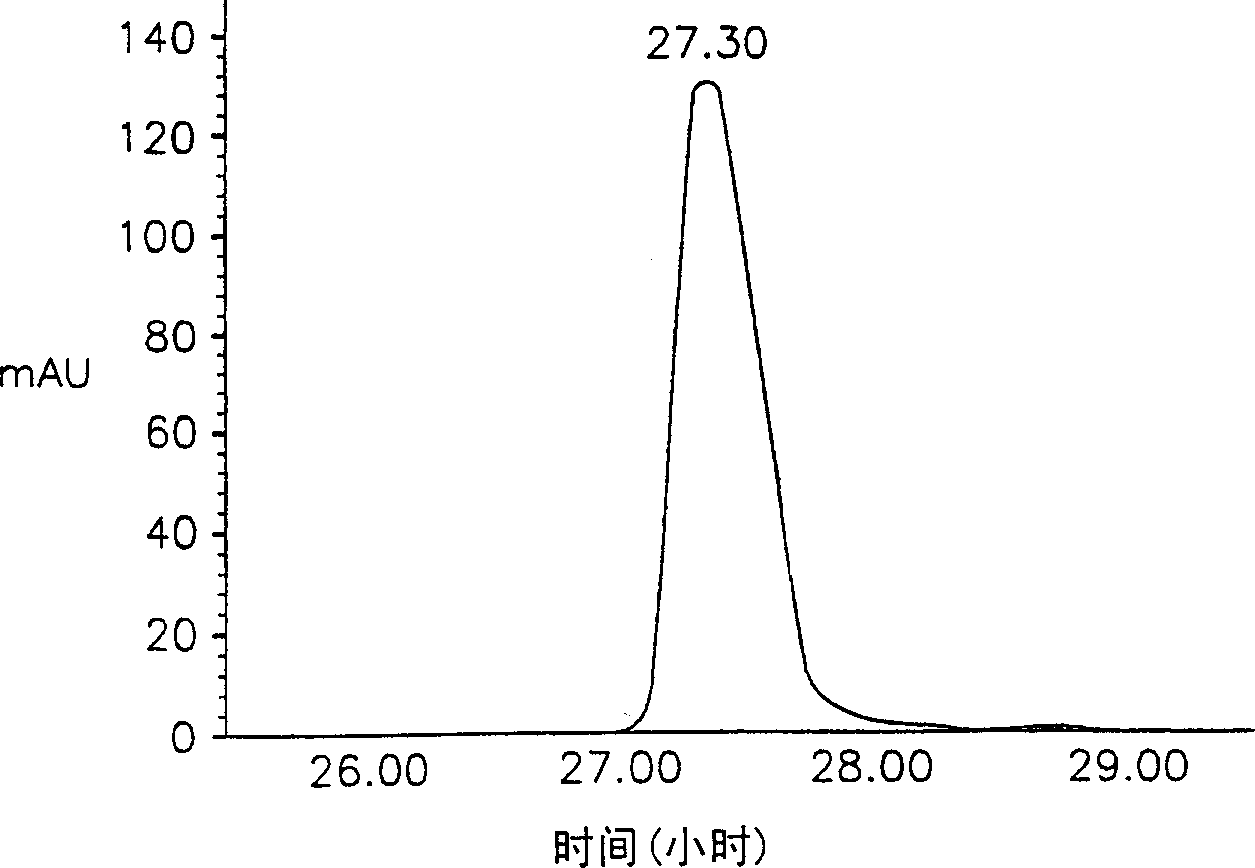
[0038] Lecithin was added to 10 mM phosphate buffered saline (PBS) at a concentration of 2% (w / v) and fully hydrated. Recombinant hepatitis B surface antigen (HBsAg, LG Chemical Ltd.) was added thereto to make the concentration 0.5 mg / ml, and then the resulting solution was sent into a spray dryer (Buchi 191), solid particles (particle 1) were obtained. In this step, the inflow air temperature was 70°C and the outflow air temperature was 50°C. The particles thus obtained have a particle size between 0.1-5 μm. Example 2: Preparation of lipophilic microparticles
Embodiment 2
[0038] Lecithin was added to 10 mM phosphate buffered saline (PBS) at a concentration of 2% (w / v) and fully hydrated. Recombinant hepatitis B surface antigen (HBsAg, LG Chemical Ltd.) was added thereto to make the concentration 0.5 mg / ml, and then the resulting solution was sent into a spray dryer (Buchi 191), solid particles (particle 1) were obtained. In this step, the inflow air temperature was 70°C and the outflow air temperature was 50°C. The particles thus obtained have a particle size between 0.1-5 μm. Example 2: Preparation of lipophilic microparticles
[0039] Recombinant HBsAg was dissolved in 10 mM PBS to a concentration of 0.5 mg / ml, and then carboxymethylcellulose was added thereto to a concentration of 3% (w / v). The resulting solution was fed into a spray dryer (Buchi 191) at a low flow rate of 0.55 ml / min to obtain primary granules. In this step, the inflow air temperature was 70°C and the outflow air temperature was 50°C.
[0040] An ethanol solution conta...
Embodiment 3
[0041] Carboxymethylcellulose was dissolved in 10 mM PBS to make the concentration 3% (w / v). To this was added lecithin to a concentration of 2% (w / v) and fully hydrated. Then, recombinant HBsAg was added thereto to make the concentration 0.5 mg / ml. The resulting solution was fed into a spray drier (Buchi 191) at a flow rate of 0.55 ml / min to obtain solid microparticles (microparticle 3). In this step, the inflow air temperature was 70°C and the outflow air temperature was 50°C. The particle size thus obtained is between 0.1-5 μm. Examples 4-9: Preparation of lipophilic microparticles
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com