Catalyst for autothermal reformation of methanol to prepare hydrogen and its prepn process and application
A technology of autothermal reforming and catalysts, which is applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, etc., to achieve the effects of easy replacement, small heat capacity and compact structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
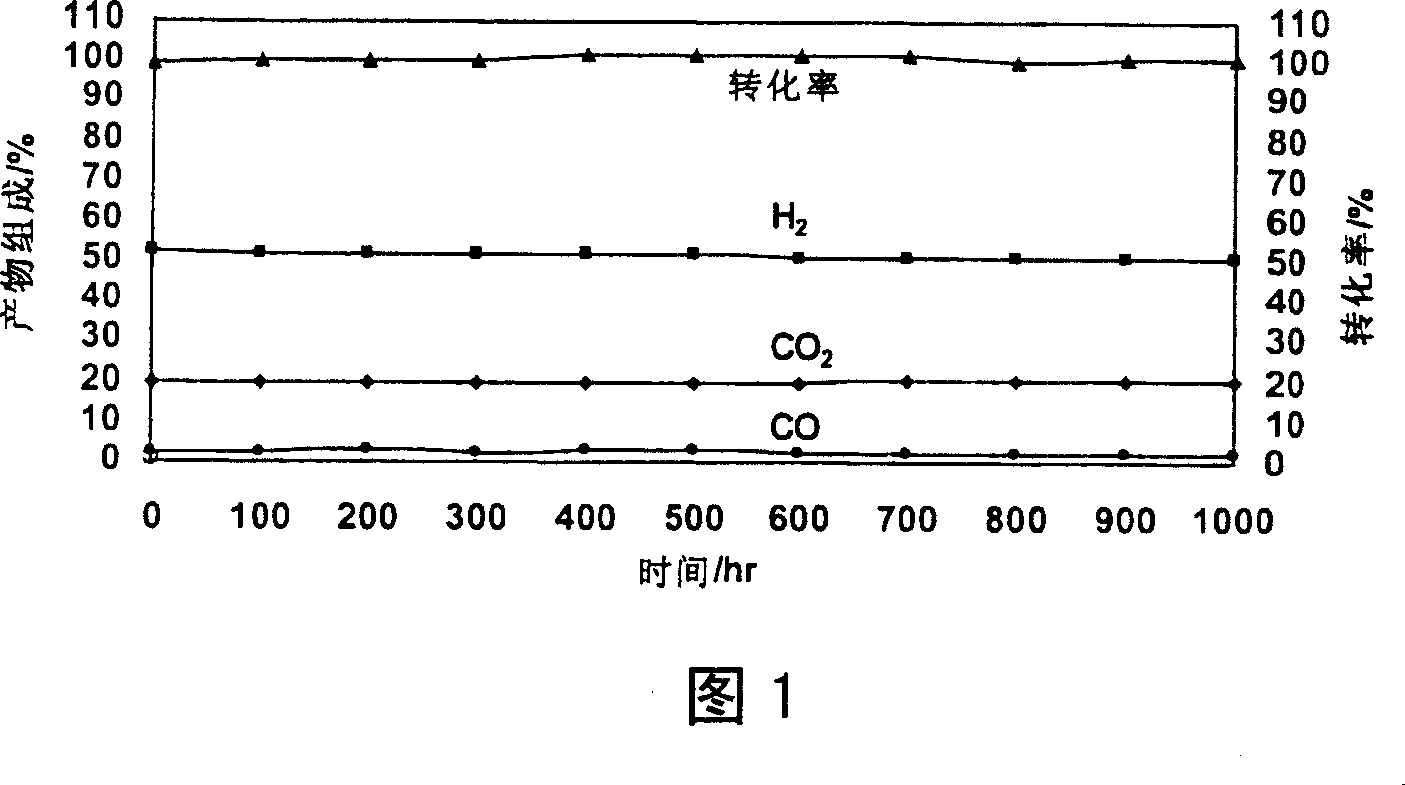
[0039] Example 1: Preparation of Zn-Cr / Ce-Zr / cordierite catalyst
[0040] a) Take 1 piece of φ15×25mm 400cpsi (400 holes / square inch) cordierite honeycomb ceramics and use 3% HNO 3 Pretreatment for 12 hours, drying at 120°C for 12 hours, and roasting at 900°C for 2 hours for later use.
[0041] b) Weigh industrial grade Ce(NO 3 ) 3 ·6H 2 O 630g, add deionized water and stir to dissolve. Weigh industrial grade Zr(OH) 4 57.8g, put it into a beaker, add 110±2ml of 65-68% concentrated nitric acid, heat the reaction until there are no visible particles and the solution is transparent, add 200±10ml of deionized water, and the solution becomes clear. The dissolved Zr(NO 3 ) 4 The solution was poured into Ce(NO 3 ) 3 solution, filtered, stirred and mixed evenly to obtain Ce(NO 3 ) 3 with Zr(NO 3 ) 4 mixture. With 13% NH 4 OH as gelling agent, with 35% HNO 3 As a debonding agent, CeO was prepared by the sol-gel method 2 -ZrO 2 Composite oxide sol is for use. The mea...
example 2
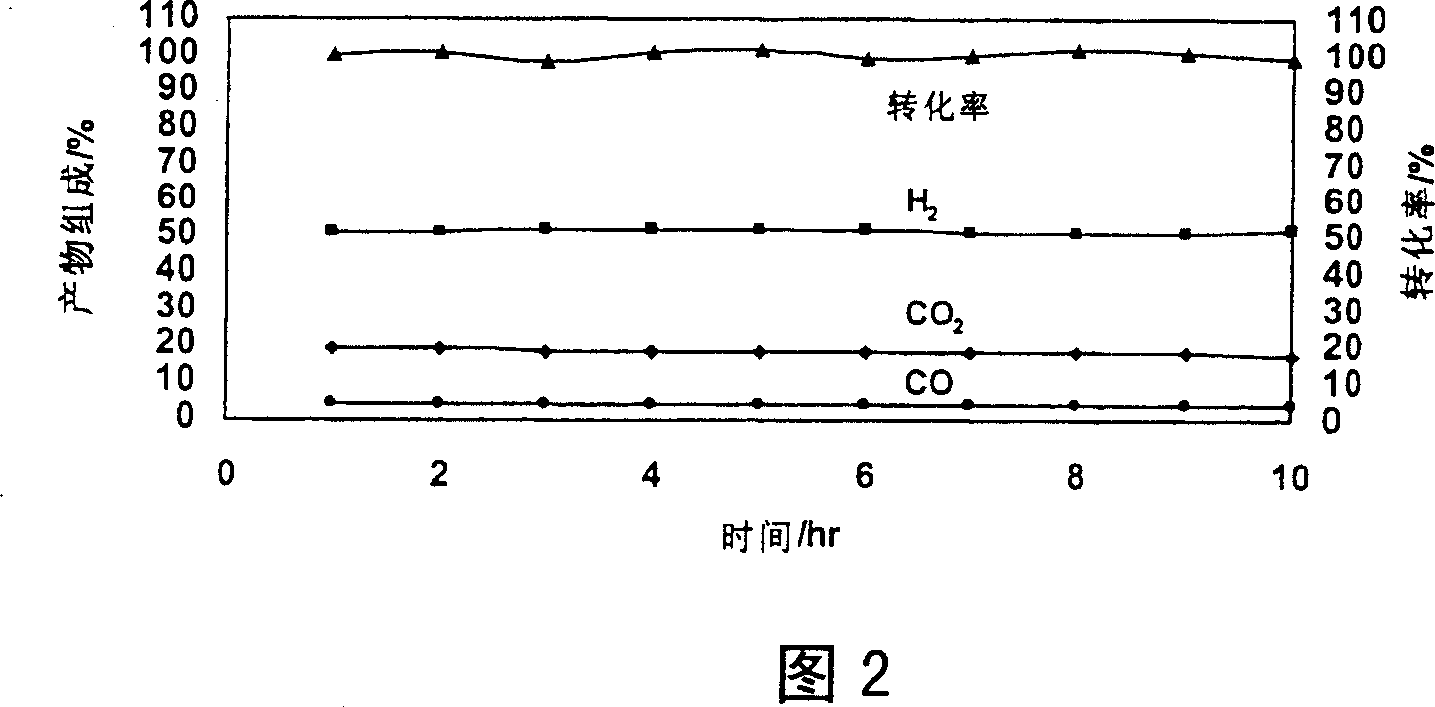
[0045] Example 2: Preparation of Zn-Cr / Ti-Zr / cordierite catalyst
[0046] a) The pretreatment of the cordierite honeycomb ceramic carrier is the same as in Example 1.
[0047] b) Weighing analytically pure TiO 2 Powder 42.7535g, analytically pure ZrO 2 Powder 42.5780g, add 40ml deionized water and 4g pseudo-boehmite (Al 2 o 3 ·H 2 O), ball milled for 18 hours. The ball mill slurry is then adjusted to a pH value of 3.0 with 10% dilute nitric acid solution for later use.
[0048] c) Weigh the above-mentioned honeycomb ceramic carrier weight 2.2191g, with TiO 2 -ZrO 2 The composite oxide latex was immersed for 3 minutes, and after taking it out, the channel was purged with compressed air, dried in microwave for 3 minutes, and calcined in a muffle furnace at 500°C for 2 hours to obtain a catalyst intermediate weight of 2.6634g.
[0049] d) Measure 70ml of the Zn-Cr mixed solution of Example 1, move it into a beaker and place it on a temperature-programmed electric furnace ...
example 3
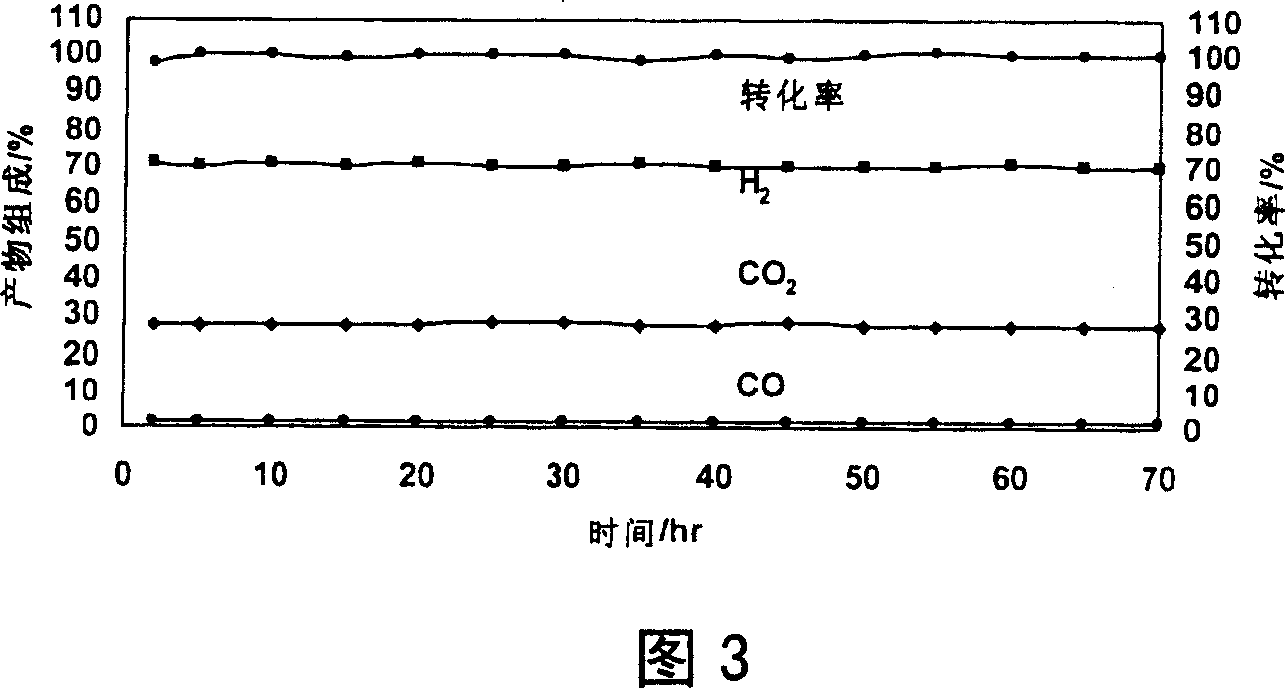
[0050] Example 3: Zn-Cr / Ce-Zr / Al 2 o 3 / cordierite catalyst preparation
[0051] a) The pretreatment of the cordierite honeycomb ceramic carrier is the same as in Example 1.
[0052] b) Weigh pseudo-boehmite (Al 2 o 3 ·H 2 O) 9.5g, gibbsite (Al(OH) 3 ) 12.4g, aluminum oxide (γ-Al 2 o 3 ) 14.3g, aluminum nitrate Al(NO 3 ) 3 9H 2 06.7g, after mixing, add 250ml of deionized water, 5ml of 65-68% nitric acid, ball milled for 12 hours to obtain an aluminum latex (slurry) with an average particle diameter of 1 μm, and record its viscosity as 1300 centipoise (cp), and the pH is 3.60.
[0053] c) Take 1 piece of pretreated carrier, weighing 1.4834g, impregnate it with aluminum latex for 3 minutes, blow the channel with compressed air after taking it out, dry it in microwave for 3 minutes, and roast it in a muffle furnace at 650°C for 2 hours to obtain the weight of the catalyst intermediate 1.5385g.
[0054] d) CeO 2 -ZrO 2 Composite oxide sol preparation and coating ar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com