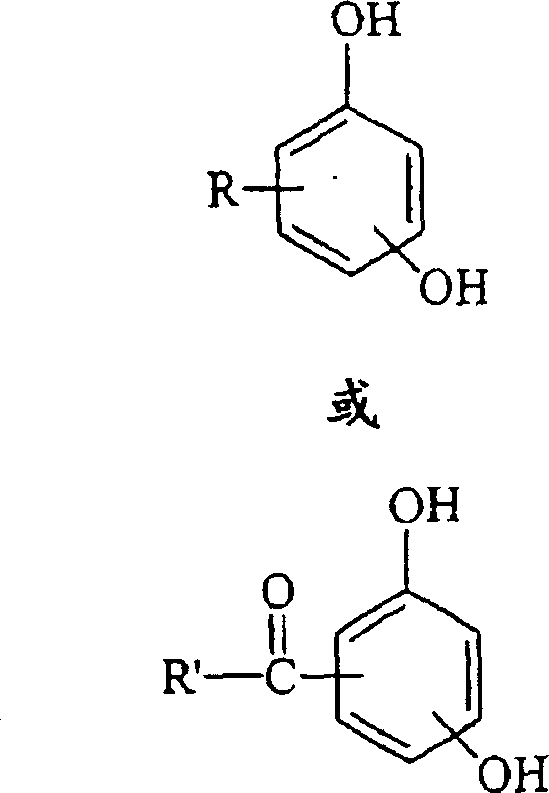
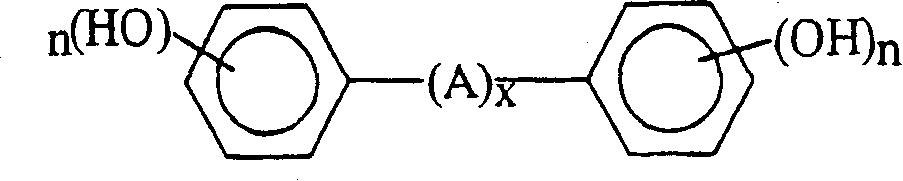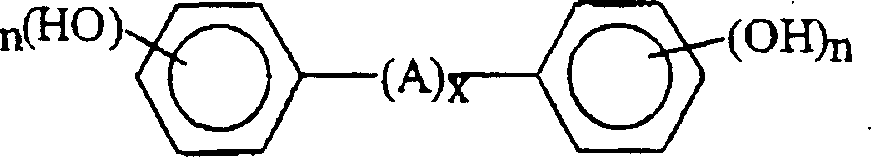Curable base-resistant fluoroelastomers
一种弹性体、单体的技术,应用在多羟基可固化含氟弹性体组合物领域,能够解决工业过程不适合、过氧化物固化不理想、可固化组合物易焦烧等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The inventive polymer (Polymer 1) was prepared by semi-batch emulsion polymerization carried out at 60°C in a well stirred reaction vessel. 20 liters of deionized deoxygenated water, 150 grams of ammonium perfluorooctanoate and 70 grams of disodium hydrogen phosphate heptahydrate were added to a 33 liter horizontally stirred reactor. The reactor was heated to 60°C and then filled with 96 wt% tetrafluoroethylene (TFE), 2 wt% propylene (P) and 2 wt% CF 3 CF 2 CH=CH 2 The mixture of curing site monomers was pressurized to 1.93 MPa. A 250 mL aliquot of a 10% by weight aqueous ammonium persulfate initiator solution was then added. The reactor was fed with 70.0 wt% TFE, 20.0 wt% P and 10.0 wt% CF 3 CF 2 CH=CH 2 mixture to maintain a pressure of 1.93 MPa throughout the polymerization. The initiator solution was fed continuously at 5 ml / h until the end of the reaction period. After a total of 6000 grams of monomer mixture had been fed to the reactor, the monomer feed wa...
Embodiment 2
[0056] A curable composition of the invention (Sample 1) was produced by mixing the following ingredients: 100 parts by weight per hundred rubber (phr) Fluoroelastomer of the invention (Polymer 1 prepared above), 2 phr Bisphenol AF, 6 phr Maglite (R) D Magnesium Oxide (available from C.P. Hall), 2.3 phr Tetrabutylammonium Hydroxide and 30 phr MT Carbon Black on a conventional two roll rubber mill using standard mixing techniques used in the elastomer industry. A comparative curable composition (Comparative Sample A) was produced by the same procedure except using 3 CF 2 CH=CH 2 A prior art fluoroelastomer (Comparison Polymer A prepared above) was cured in monomeric units.
[0057] Curing properties were determined by MDR (24 minutes at 177° C.) according to the test method. Sample 1 cured as indicated by the increase in torque (M) during the test. However, control polymer B, which does not contain CF 3 CF 2 CH=CH 2 The copolymerized units of the cure site monomers do no...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal degradation temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com