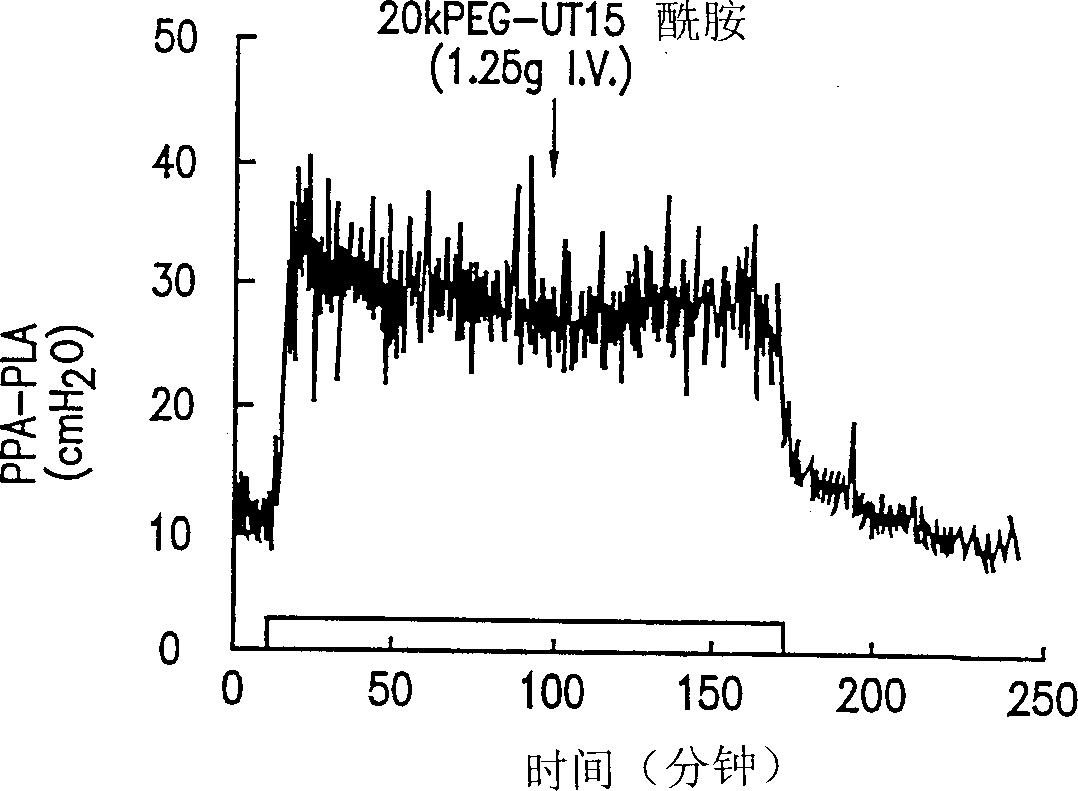
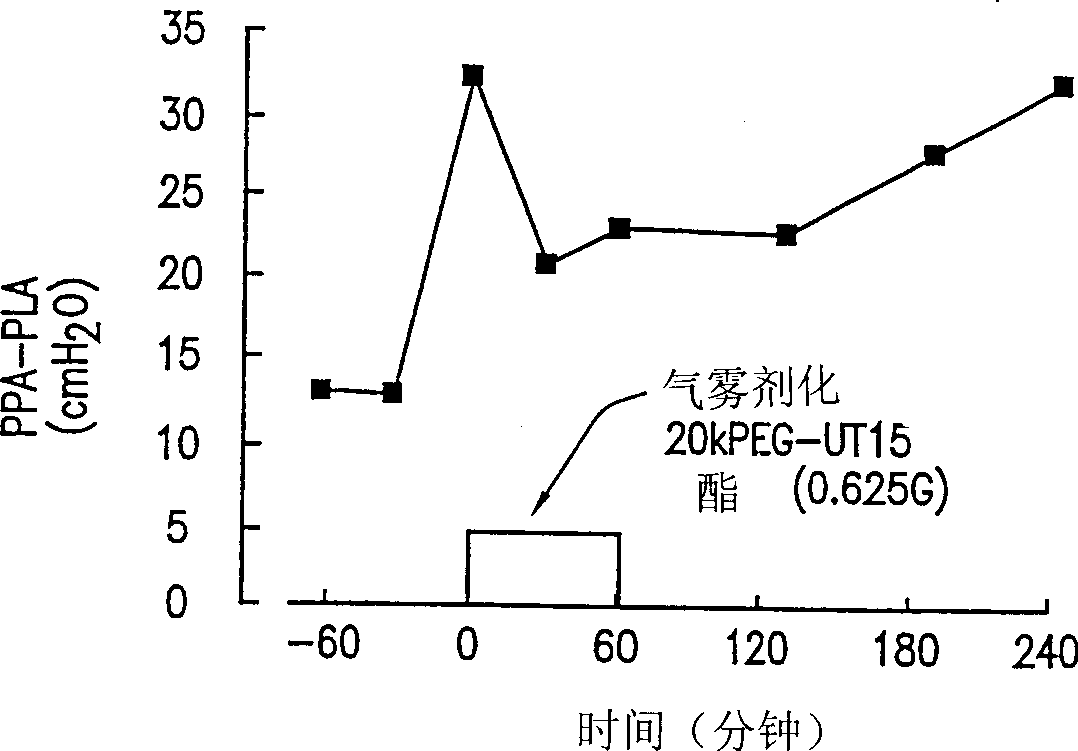
Prostaglandin compound, compositions and methods of treating peripheral vascular disease and pulmonary hypertension
A prostaglandin and compound technology, applied in the field of modified prostaglandin compounds, can solve the problems of patients, short effective life, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] Synthesis of mPEG-5kDa-amide-Compound X
[0103] Hereinafter referred to as "Compound 1"
[0104] Compounds of group 4 were prepared in the following manner, wherein Z 1 is mPEG with a molecular weight of approximately 5,000 Daltons, X is NH and Z 2 for hydrogen.
[0105] 200 mg of Compound X having the following structural formula:
[0106]Placement containing mPEG5k amine (2.5g), 2-hydroxybenzyltriazole (HOBT, 67mg), 4-(dimethylamino)pyridine (DMAP, 61mg) and dicyclohexylcarbodiimide (DCC, 140mg) inside the round bottom flask. The reactants were mixed with 60 ml of anhydrous dichloromethane. The mixture was stirred at room temperature overnight, after which time the solvent was evaporated. The residue was dissolved in 25 ml of 1,4-dioxane, and the insolubles were removed by filtration. The solvent was concentrated and then precipitated into 100 ml of 50:50 / diethyl ether:isopropanol. The precipitate was collected by filtration and dried under vacuum. The pro...
Embodiment 2
[0108] Synthesis of mPEG5kDa-ester-compound X diacetate
[0109] Hereinafter referred to as "Compound 2"
[0110] Compounds of group 4 were prepared in the following manner, wherein Z 1 is mPEG with a molecular weight of approximately 5,000 Daltons, X is O and each Z 2 for the acetyl group.
[0111] In a round bottom flask, compound X (400 mg) and pyridine (200 μl) were mixed in 35 ml of dry dichloromethane, and 500 μl of acetic anhydride was added to the suspension. The compound became homogeneous within a few hours and the solution was stirred at room temperature overnight. The solvent was concentrated, and phosphate buffer (0.1 M, pH 7.4) was added to the residue. The mixture was stirred rapidly for 30 minutes, and the mixture was extracted 3 times with dichloromethane. The combined organic phases were dried over sodium sulfate and the solvent was evaporated. An oily product was obtained, Compound X diacetate. The yield was 340 mg (80%). 1 H NMR (DMSO-d 6 ): 1.91(s...
Embodiment 3
[0114] Synthesis of mPEG20KDa-ester-compound X
[0115] Hereinafter referred to as "Compound 3"
[0116] Compounds of group 5 were prepared in the following manner, wherein each Z 2 is -CO-(CH with a molecular weight of about 20,000 Daltons 2 ) 2 -O-linked mPEG.
[0117] In a round bottom flask, compound X (200 mg) and sodium hydroxide (21 mg) were mixed in 40 ml of anhydrous acetonitrile. To the suspension was added 90 mg of benzyl bromide, and the mixture was refluxed for 2 days. The solids were removed by filtration, the solvent was concentrated, and the residue was dried under vacuum. An oily product is obtained, compound X-benzyl ester. The yield is 210mg (100%). 1 H NMR (DMSO-d 6 ): δ7.37(s, C 6 H 5 -CH 2 -OCO-(Compound X)), 5.19(s, C 6 H 5 -CH 2 -OCO-(Compound X)), 4.83(s, (Compound X)-CH 2 COOBz), 4.49(d, (Compound X)-OH 1 ), 4.24(d, (Compound X)-OH 1 ), 0.864(t, (Compound X)-CH 3 ), 7.025 (t, compound X aromatic proton), 6.7 (d+d, compound X aromatic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com