Dry powder inhaler devices, multi-dose dry powder drug packages, control systems, and associated methods
A dry powder inhaler, dry powder technology, applied in inhalers, drug devices, therapeutic insufflators, etc., can solve problems such as drug residues, the influence of stable powder distribution, and difficulty in reducing the amount of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
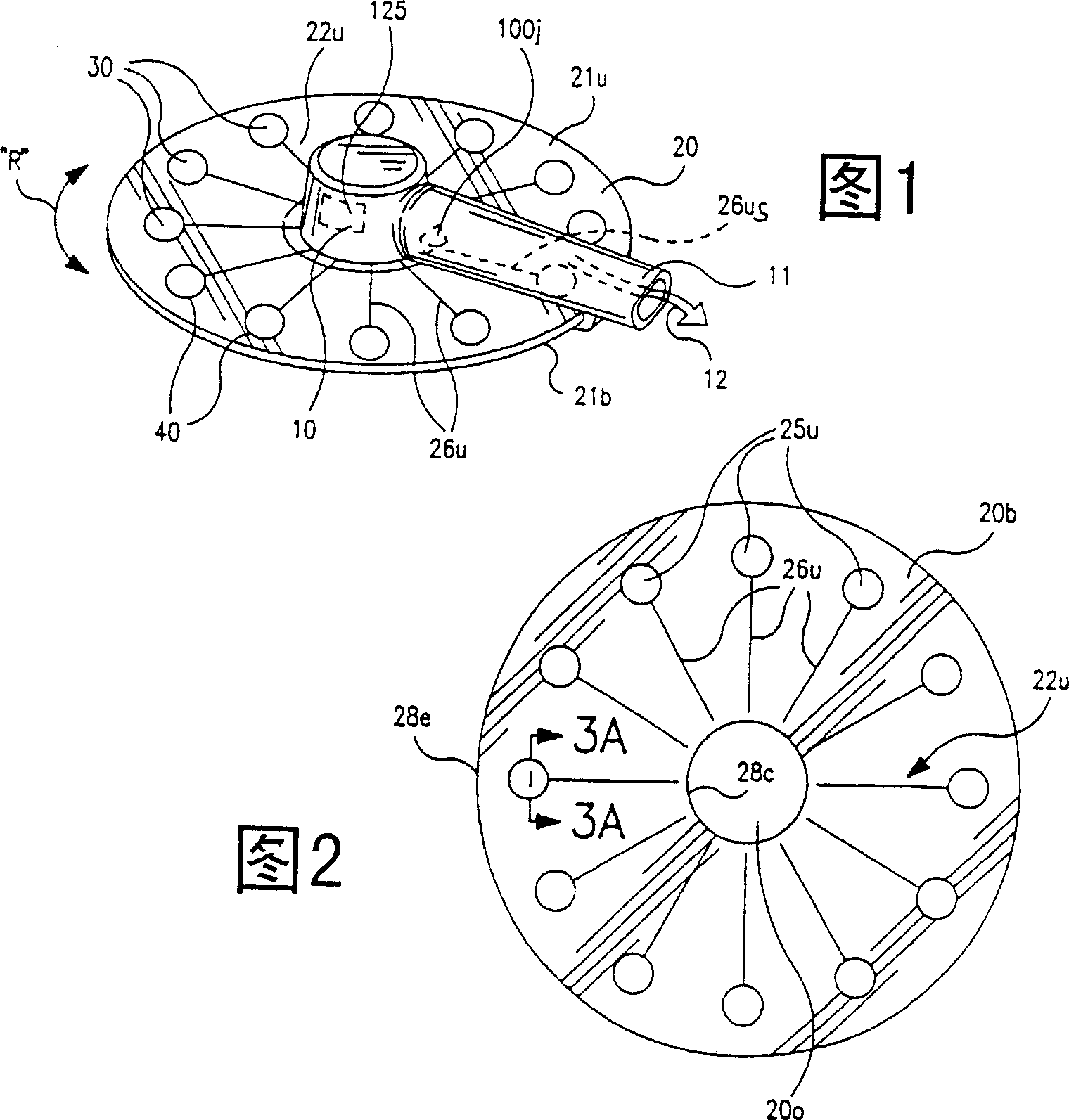
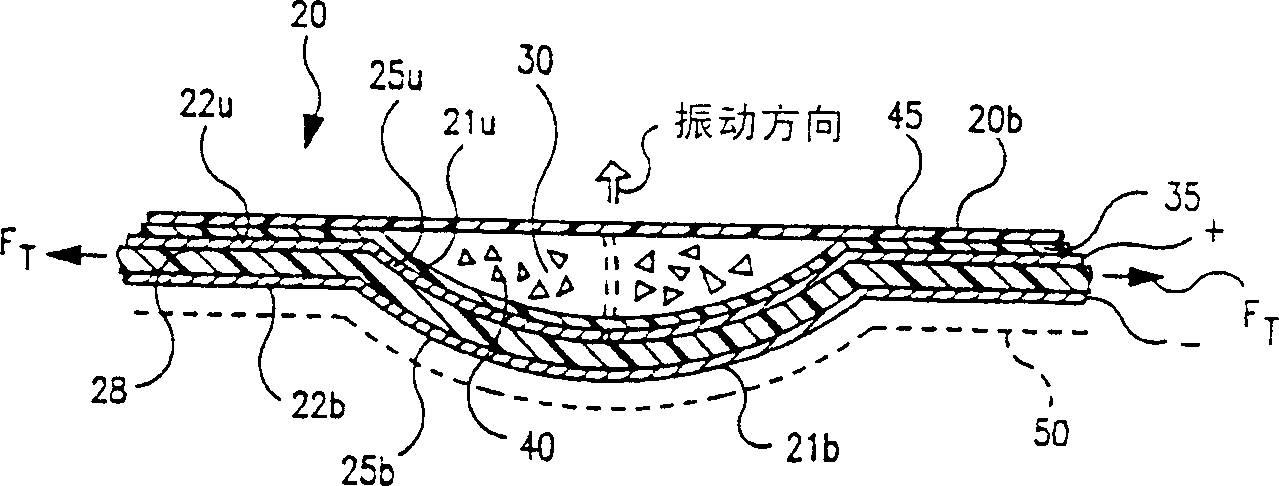
[0131] In an experimental DPI embodiment, a piezoelectric excitation element for vibrating the powder during dispensing was employed, the DPI having a structure such that the polymer film vibration element had an associated capacitance C greater than 1800 pf. This capacitance value is related to the size or area (and thus shape) of the capsule or vibrating element. The transformer used to boost the input voltage of 5Vp-p has an inductance of about 23mH on its second side. A transformer is used to step up the voltage to the capsule excitation voltage of 150Vp-p. In this way, the transformer and the piezoelectric element are combined to form an amplifier, and the resonant frequency of the amplifier can be expressed as the following equation:
[0132] f=1 / (2π(LC) 1 / 2 )
[0133] Among them, L is the inductance of the transformer, and C is the capacitance of the polymer film vibration element. This results in a calculated resonant frequency of about 25 kHz for the experimental ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap