Infant formulas contg. long-chain polyunsaturated fatty acids and uses thereof
An infant formula and fatty acid technology, applied in the field of nutritional formula, can solve problems such as insufficient full-process synthesis, limited supply, and slow growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
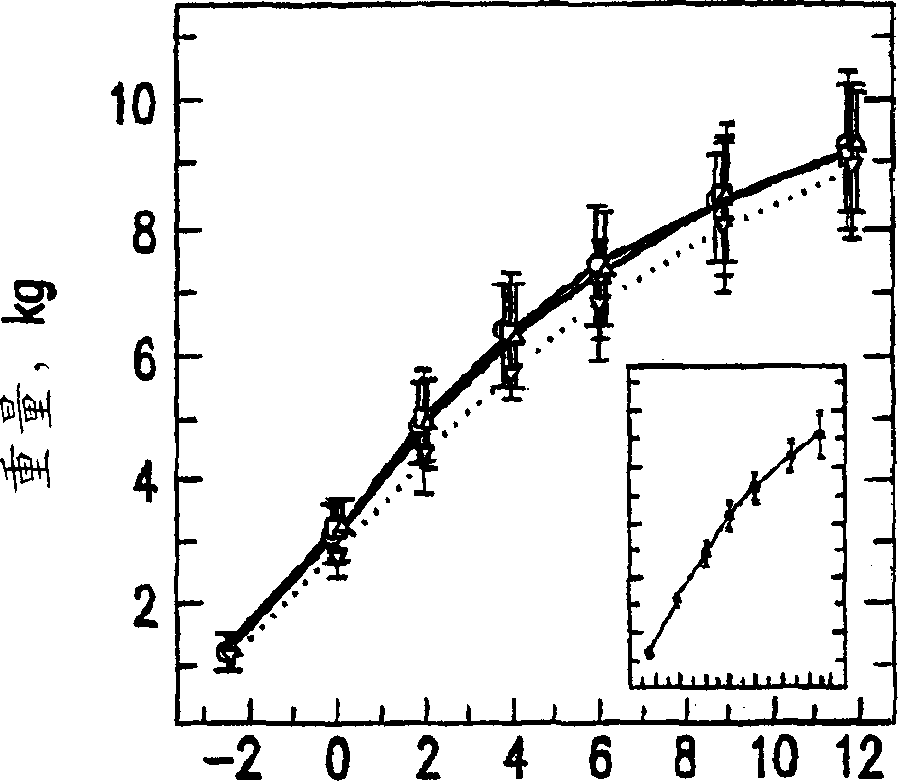
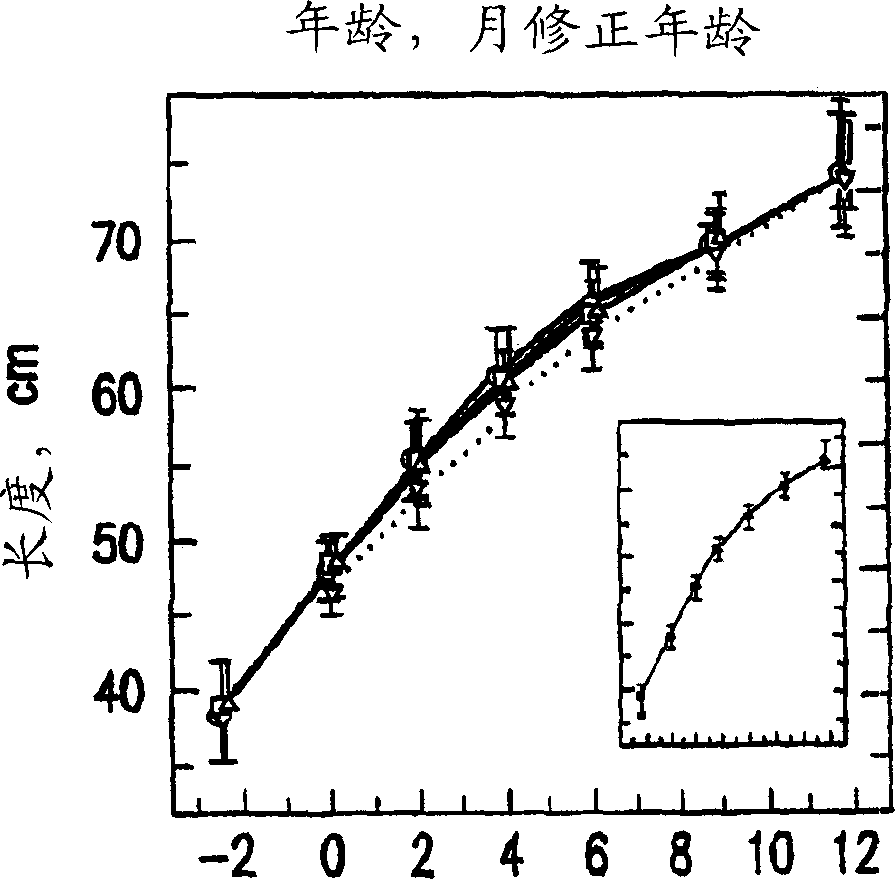
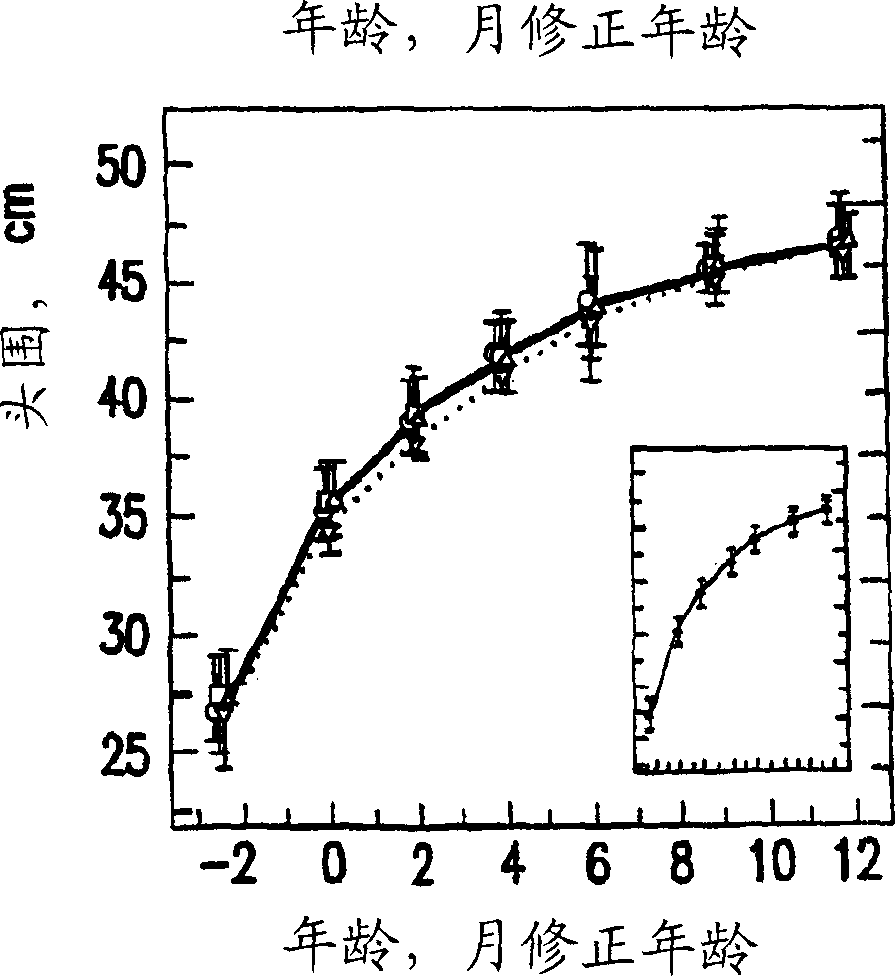
[0137] Embodiment 1: clinical research 1.1 research sample selection
[0138] Four hundred and seventy preterm infants ( grade II, maternal insufficiency including maternal cocaine or alcohol abuse or both during pregnancy, extracorporeal membrane oxidation, fluid ventilation, asphyxia due to severe and permanent neurological injury , or have uncontrolled systemic infection at the time of enrollment. In addition to the criteria of registration within 72 hours of the first enteral feeding, 8% of the subjects were registered outside this specified window, and 99% of the study subjects met all the specified entry and exclusion criteria. 1.2 Experimental design
[0139] 1.2.1 Research formula
[0140] According to the conventional process mentioned above, the nutrient-enriched formula containing DHA and AA was prepared. Modified formulations of SIMILAC SPECIAL CARE (type 1) and SIMILAC NEOSURE (type II) used in the present study differed from the formulations of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com