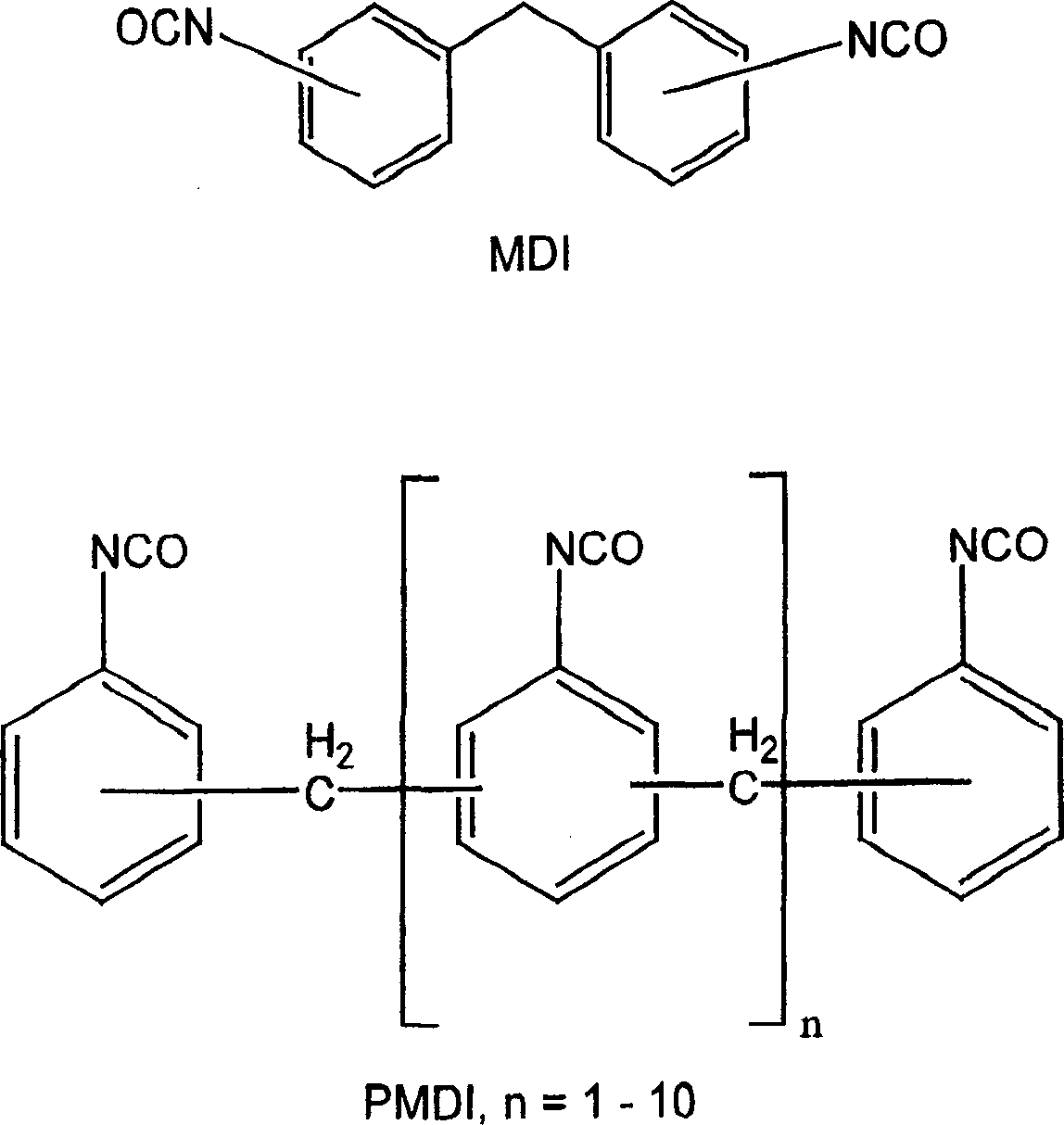
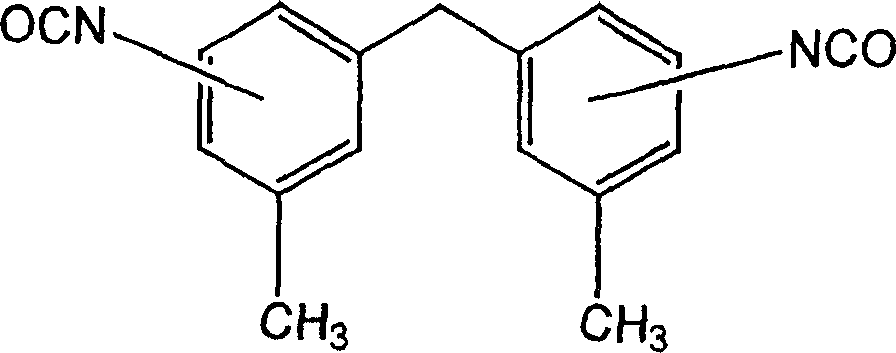
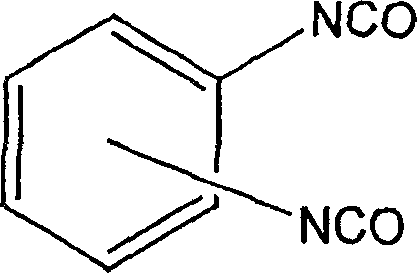
Method for synthesis of aliphatic isocyanates from aromatic isocyanates
A technology of aromatic isocyanate and isocyanate, which is applied in the preparation of isocyanic acid derivatives, carbamate preparation, organic chemical methods, etc., can solve the problems of cost, output loss, catalyst passivation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0118] has about 33m 2 Alumina shaped body (extrusion, d=3mm) with BET surface area and pore volume of 0.41ml / g bimodal pore distribution (extrusion, d=3mm) in which basically no pores with a diameter of 2-50nm were detected, while 100% of the pore volume Consisting of macropores with a diameter in the range of 50-10000nm, the alumina-shaped body is coated with an aqueous solution of ruthenium(III) nitrate at 90-100°C, in which the catalyst solution is sprayed on a movable carrier while the water is evaporated .
[0119] The catalyst solution has a concentration of 5% metal, based on the weight of the solution. The support coated in this way is heated at a temperature of 120-180 °C and subsequently treated with 50% H 2 and 50% of N 2 The mixture was reduced at 200 °C for 4 h. The catalyst prepared in this way contained 3% by weight of ruthenium, based on the total weight of the catalyst. The penetration depth of ruthenium is 70-90 μm. The ratio of the ruthenium surface a...
Embodiment 2
[0121] The composition is similar to the carrier of Example 1 and has about 32m 2 An alumina shaped body (extrudate, d=3 mm) with a BET surface area per g, a trimodal pore distribution and a pore volume of 0.58 ml / g was impregnated in a similar manner to Example 1. 31% of the pore volume of the support material consists of pores of 2-50 nm, 44% of pores of 50-10000 nm and 25% of pores with a pore diameter of more than 10000 nm to 5 μm. As in Example 1, the catalyst prepared in this way contained 3% by weight of ruthenium, and the penetration depth was 70-90 μm.
Embodiment 3
[0123] has about 54m 2 The alumina shaped body (extrudate, d=3 mm) of surface area per gram had a trimodal pore distribution and a pore volume of 0.77 ml / g. 40% of the pore volume consists of pores with a diameter of 2-50 nm and 60% is formed by pores with a diameter of 50-10000 nm. Impregnation of the carrier, calcination and reduction of the catalyst were carried out in the same manner as in Example 1. The catalyst prepared in this way contained 3% by weight of ruthenium, based on the total weight of the catalyst. Penetration depth is 70-90nm. The alumina forms used include α-, θ- and γ-Al 2 o 3 transform.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Penetration depth | aaaaa | aaaaa |
| Penetration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com