Method of modulating activity of calcium channels in cardiac cells and reagents therefor
A calcium channel and channel technology, which can be used in non-central analgesics, testing pharmaceutical preparations, chemical instruments and methods, etc., and can solve problems such as toxicity and calcium overload.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0164] A 20-mer peptide that modulates cardiac RYR2 calcium channel activity
[0165] Materials and methods
[0166] Material
[0167]Chemical and biological reagents were purchased from Sigma-Aldrich (Castle Hill, Australia). The DHPR II-III cyclic peptide (SEQ ID Nos: 1-7) was synthesized by Applied Biosystems 430A peptide synthesizer, and purified by HPLC, mass spectrometry and NMR with a purity of ≥98%. Peptides were prepared as ~2 mM stock solutions in water and frozen in 20 [mu]l aliquots. The exact mother liquor concentration was determined by Auspep Pty Ltd using acid hydrolysis followed by standard PTC (phenylthiocarboxamido) method and analyzed by reverse phase HPLC.
[0168] peptide
[0169] The specific peptides used for the study were:
[0170] 1. 20-mer peptide of DHPR II-II cytoplasmic loop (SEQ ID NO: 2):
[0171] Thr Ser Ala Gln Lys Ala Lys Ala Glu Glu Arg Lys Arg Arg Lys Met Ser Lys Gly Lea
[0172] 2. Peptide NB (N-...
Embodiment 2
[0189] A 20-mer peptide that modulates cardiac RyR2 calcium channel activity
[0190] result
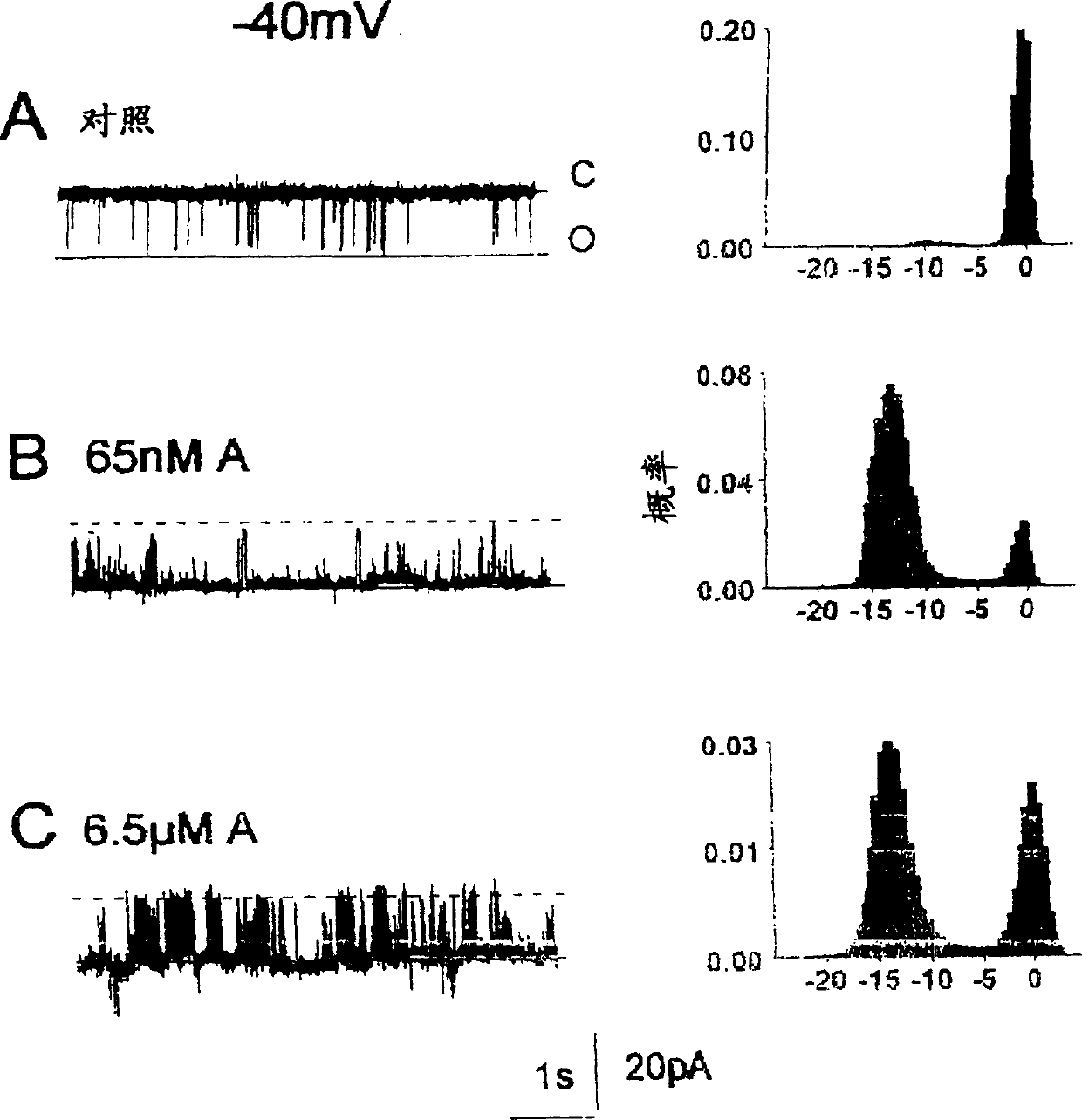
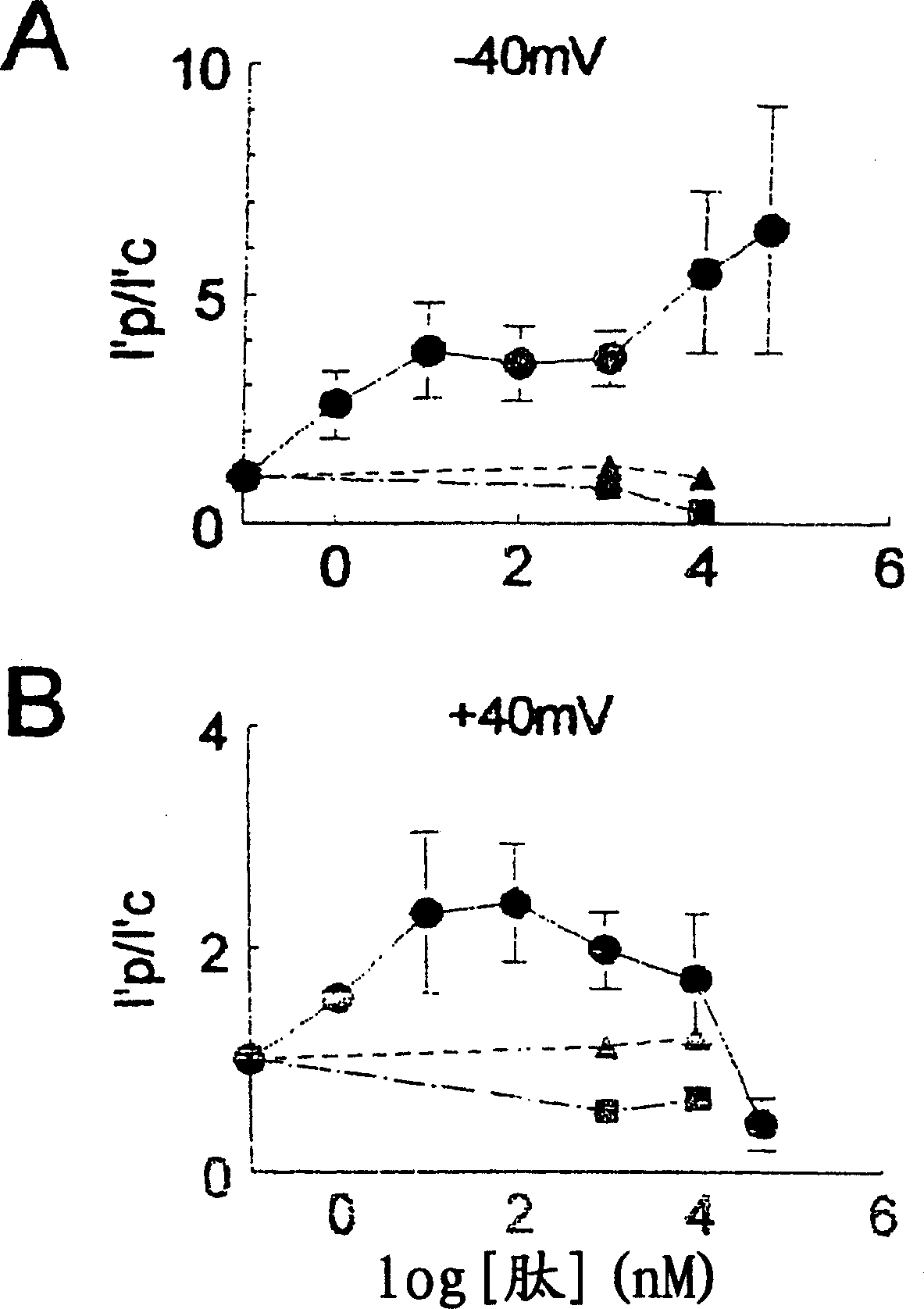
[0191] When the concentration is 10 -7 M cis Ca 2+ , the addition of a 20-mer peptide (SEQ ID NO: 2) to the cytoplasmic (cis) side of the channel observed an increase in the activity of myocardial RyRs. Cs around 450pS at +40mV or -40mV double layer potential + The ability to conduct and block channels at the end of the assay with 30 μM ruthenium red identified single channels as RyRs.
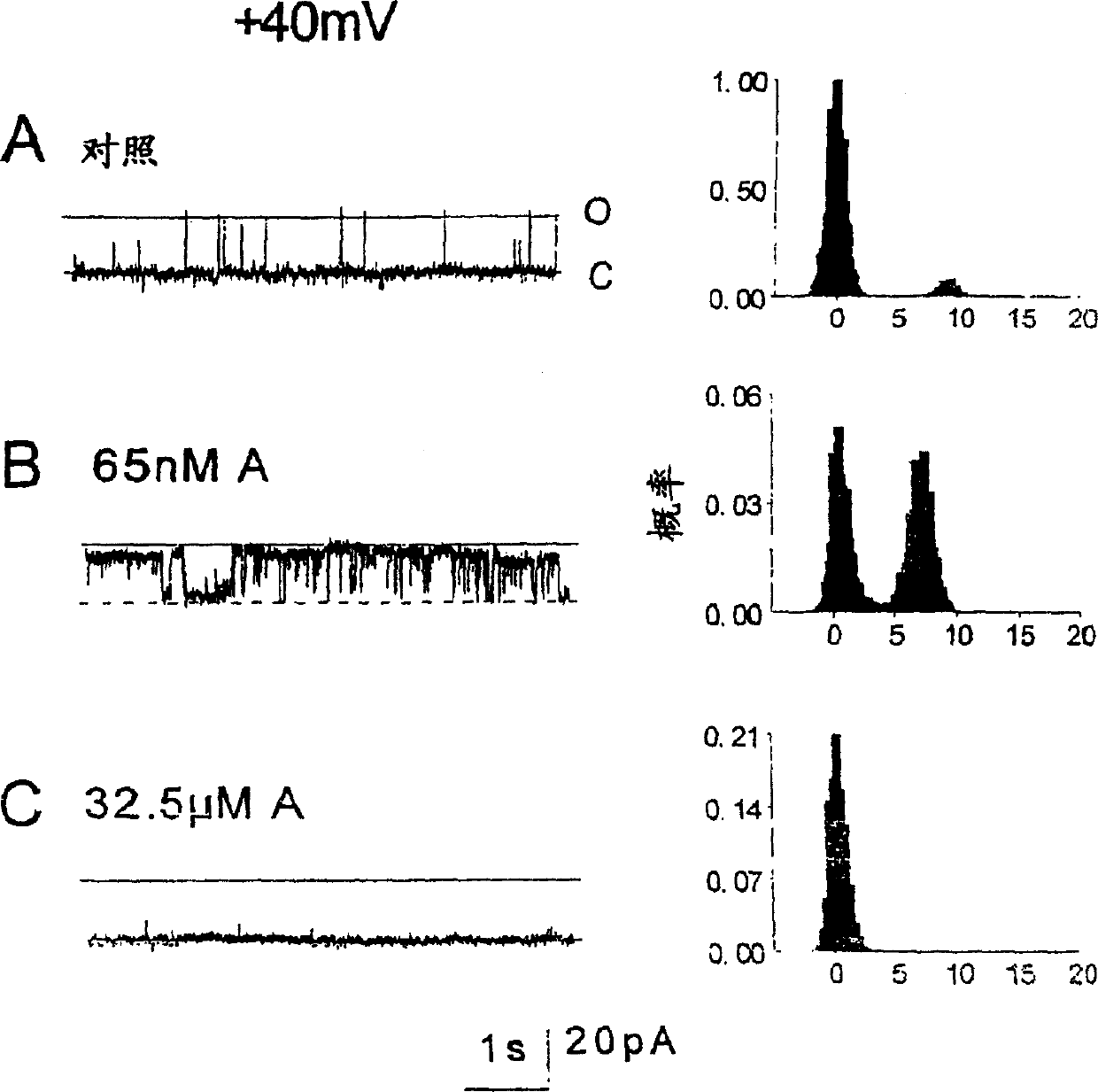
[0192] Figure 2 shows recordings from an experiment in which cardiac RyRs were strongly activated by a 20-mer peptide (SEQ ID NO: 2) at -40 mV and Figure 3 at +40 mV. Before peptide addition, channel activity consisted of brief intermittent openings (Fig. 2, panel A; Fig. 3, panel A). Channel opening increased at -40 mV within 10 s of addition of 65 nM peptide (SEQ ID NO: 2).
[0193] The opening of the second channel in the bilayer was accompanied by an incr...
Embodiment 3
[0203] Functional Analysis of DHPR 20-MER Fragment Derivatives and Analogs
[0204] Materials and methods
[0205] peptide
[0206] Four peptides were tested in this series of experiments and they are:
[0207] (i) native DHPR 20-mer peptide (SEQ ID NO: 2);
[0208] (ii) Ser in the peptide of SEQ ID NO: 2 687 (residue 17) was substituted with an alanine residue to generate SEQ ID NO: 8;
[0209] (iii) Arg in the peptide of SEQ ID NO: 2 688 (residue 18) is replaced by the D isomer to generate SEQ ID NO: 9; and
[0210] (iv) Ser in the peptide of SEQ ID NO: 2 687 Mutation to alanine, Arg 688 Substitution to the D isomer yielded SEQ ID NO:10.
[0211] from cardiac SR Ca 2+ Determination of release
[0212] Add cardiac SR vesicles (50 μg protein) to a cuvette to a final volume of 2 ml solution containing (in mM): 100, KH 2 PO 4 (pH=7); 4, MgCl 2 ; 1, Na 2 ATP; 0.5, antipyrylazo III. Use Cray50 or Cray100 spectrophotometer to monitor ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com