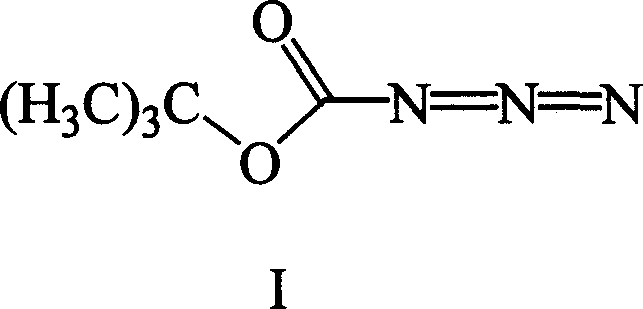
Amino protective reagent azido tert.-butyl formate synthesizing method
A technology of amino protection and synthesis method, which is applied in the direction of organic chemistry, can solve the problems of cumbersome synthesis route and not widely used, and achieve the effect of simple synthesis route, low toxicity and less side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Add 52.5 grams of tert-butanol (0.71 moles) in a 500 milliliter three-necked flask, 55 milliliters of pyridine (0.71 moles) and 110 milliliters of chloroform, ice water cooling (0 ℃), add ethyl chloroformate under stirring in 2 hours ( 0.71 mol). The reaction produced a large amount of precipitate. The reaction was continued for 24 hours. 83 g of pale yellow oily liquid (CH 3 ) 3 COCOO Et.
[0040] The product (CH 3 ) 3 COCOOEt (64g, 0.44mol) was placed in a three-necked flask, and 25ml of 85wt% hydrated NH 2 NH 2 (0.44 mol) solution, refluxed at 110°C for 12 hours. 46 g of a waxy solid (CH 3 ) 3 COCONHNH 2 .
[0041] Weigh 9.9 g (CH 3 ) 3 COCONHNH 2 (0.075 mol) was dissolved in 9 ml of acetic acid (0.15 mol) and 12 ml of water, and 6.2 g of NaNO was added dropwise at 0°C in an ice bath 2 (0.09 mol) in 10 ml of aqueous solution. The reaction was continued for 2 hours. 10 g of light yellow-green oily liquid (CH 3 ) 3COCON 3 .
[0042] IR data charac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com