Short-acting sedative hypnotic agents for anesthesia and sedation
A compound and alkyl technology, which is applied in the direction of anesthetics, active ingredients of esters, medical preparations containing active ingredients, etc., can solve problems such as short recovery time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
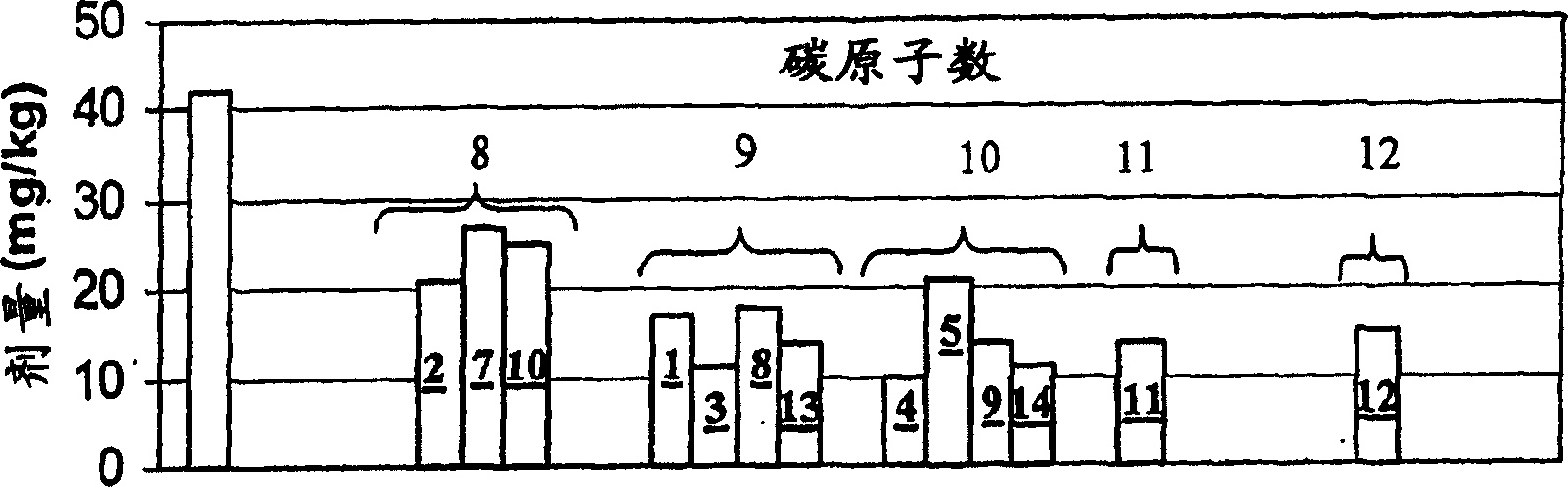
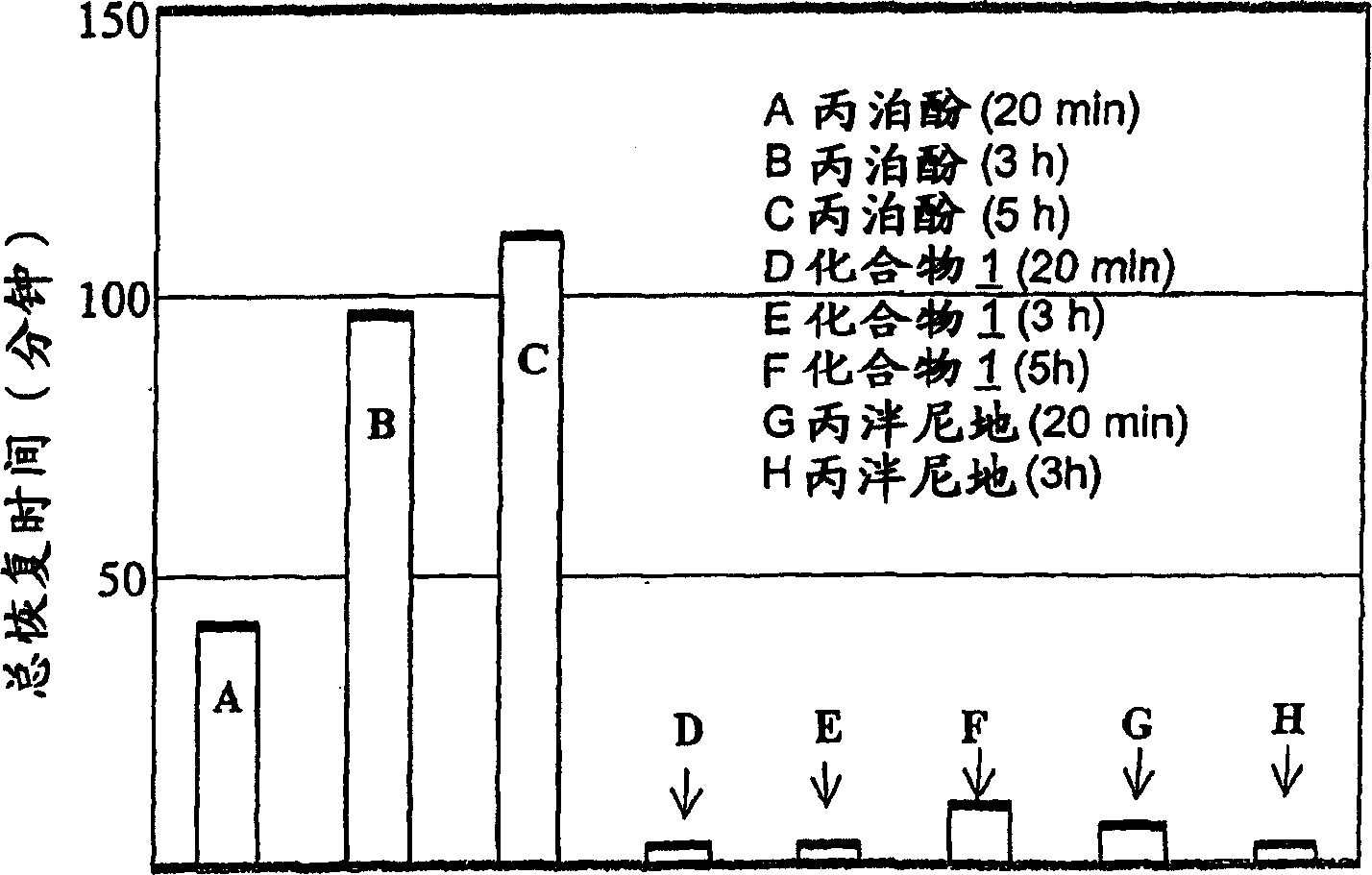
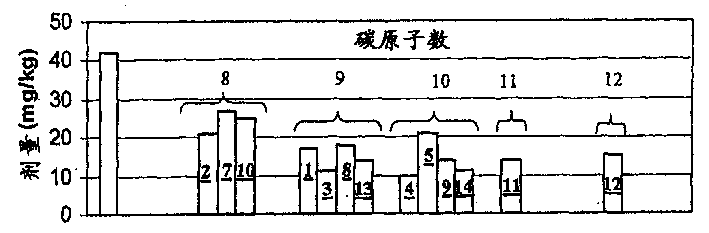
Image
Examples
Embodiment 1
[0130] Example 1. Compound 1: [4-[(N,N-diethylcarbamoyl)methoxy]-3-ethoxyphenyl]propyl acetate
[0131]
[0132] In a 50 mL round bottom flask equipped with a magnetic stir bar, propyl 3-ethoxy-4-hydroxyphenylacetate (800 mg, 3.4 mmol, 1.0 equiv.) was dissolved in anhydrous acetone (20 mL). To this solution was added K 2 CO 3 (705 mg, 5.1 mmol, 1.5 equiv.), followed by 2-chloro-N,N-diethylacetamide (0.55 mL, 4.0 mmol, 1.2 equiv., Aldrich). With vigorous stirring, the suspension was heated to reflux and maintained at this condition for 15 hours. After cooling to room temperature, the reaction mixture was filtered through folded filter paper, and the solvent of the remaining solution was removed under reduced pressure. The oily product was purified by column chromatography (SiO 2 , 50% EtOAc / Hexane) to afford 630 mg (53% of theory) of a colorless oil with a purity of 99.6% by HPLC.
[0133] TLC (Silica, 50% EtOAc / Hexane) Rf 0.25; 1 H NMR (CDCl 3 , 300MHz): d 0.90 (3H, ...
Embodiment 2
[0152] Example 2. Compound 2: [4-[(N,N-diethylcarbamoyl)methoxy]-3-ethoxyphenyl]ethyl acetate
[0153]
[0154] Utilize the technique similar to that described in Example 1, except that ethanol is used to replace 1-propanol in the synthesis of the intermediate, generate the intermediate of formula (IIa), wherein R 1 = ethyl, R 4 =Propyl, the title compound was obtained in 81% yield as a colorless oil with a purity of 96% as determined by HPLC.
[0155] TLC (Silica, 50% EtOAc / Hexane) Rf 0.25; 1 H NMR (CDCl 3 , 300MHz): d 1.13-1.22 (6H, m, N-ethyl CH 3 ), 1.25 (3H, t, ethyl ester CH 3 ), 1.43 (3H, t, ethoxy CH 3 ), 3.38-3.45 (4H, m, N-ethyl CH 2 ), 3.52 (2H, s, OCH 2 CO), 4.05-4.17 (4H, m, 2xOCH 2 ), 4.71 (2H, s, ArCH 2 CO), 6.78-6.91 (3H, m, ArH). m / z: [M+H + ]C 18 h 27 NO 5 The calculated value is 338.20; the measured value is 338.
Embodiment 3
[0156] Example 3. Compound 3: [4-[(N,N-diethylcarbamoyl)methoxy]-3-ethoxyphenyl]acetate isopropyl ester
[0157]
[0158] Utilize the technique similar to that described in Example 1, except replacing 1-propanol with isopropanol in the synthesis of the intermediate, generate the intermediate of formula (IIa), wherein R 1 = ethyl, R 4 =Isopropyl, the title compound was obtained in a yield of 63% as a colorless oil with a purity of 99% as determined by HPLC.
[0159] TLC (Silica, 50% EtOAc / Hexane) Rf 0.25; 1 H NMR (CDCl, 300MHz): d 1.06-1.19 (6H, m, N-ethyl CH 3 ), 1.14 and 1.16 (2×3H, 2s, isopropyl ester CH 3 ), 1.36 (3H, t, ethoxy CH 3 ), 3.30-3.36 (4H, m, N-ethyl CH 2 ), 3.42 (2H, s, OCH 2 CO), 3.98-4.03 (2H, m, OCH 2 ), 4.64 (2H, s, ArCH 2 CO), 4.90-4.98 (1H, m, CH), 6.71-6.84 (3H, m, ArH). m / z: [M+H + ]C 19 h 29 NO 5 The calculated value is 352.22; the measured value is 352.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com