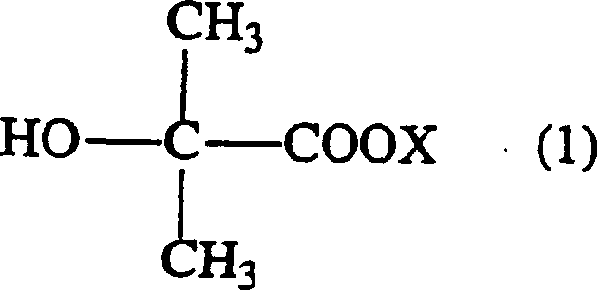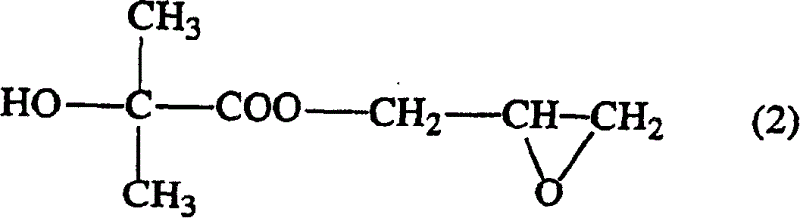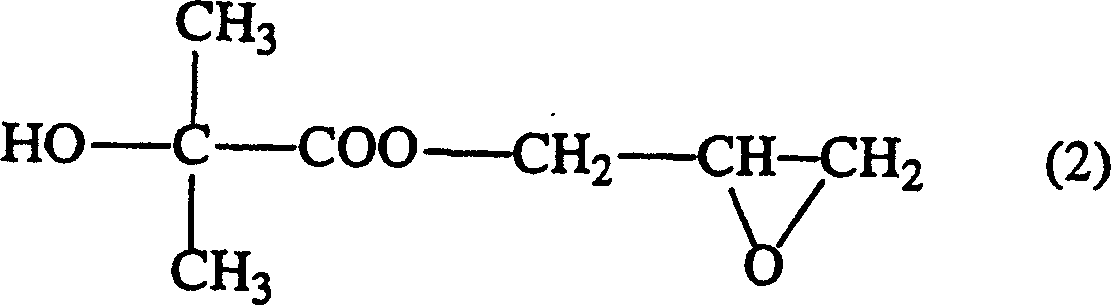Method of producing glycidyl 2-hydroxyisobutyrate
一种缩水甘油酯、羟基异丁酸的技术,应用在环氧树脂用反应性稀释剂,环氧树脂组合物领域,达到提高反应收率、容易且稳定制备、降低副产物的量的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~5 and comparative example 1
[0043] At room temperature and normal pressure, add 37.83g (0.3 mole) sodium 2-hydroxyisobutyrate, 138.7g (1.5 mole) epichlorohydrin, 0.25g tetramethyl chloride Ammonium and purified water were heated from room temperature to an internal temperature of 120° C. within 10 minutes, kept at this temperature for 1.5 hours to react, and then cooled to room temperature. After adding water therein and leaving it to stand for 1.5 hours, the organic solvent phase was taken out, epichlorohydrin was distilled off in a distillation apparatus equipped with a Vigreul distillation tube, and the resulting residue was distilled under reduced pressure to obtain 2-hydroxyisobutyric acid glycidyl esters. The mol ratio of feeding intake, reaction conditions and reaction yield, the amount of by-products after vacuum distillation are as shown in Table 1 and 2. In addition, the addition rate of the catalyst and water in a table|surface is the quantity calculated with respect to the total weight of ep...
Embodiment 1 Embodiment 2 Embodiment 3
[0046] Feeding molar ratio (formula (1) raw material compound / EpCH) 0.2 0.2 0.2
[0047] Reaction temperature (℃) 120 120 120
[0048] Reaction time (hours) 1.5 1.5 1.5
[0049] Catalyst Type TMAC TMAC TMAC
[0050] Amount added (g) 0.25 0.25 0.25
[0051] Addition rate (weight%) 0.14 0.14 0.14
[0052] Amount of water added (g) 0.55 1.00 2.00
[0053] Addition rate (weight%) 0.31 0.56 1.11
[0054] Reaction yield (%) 69 66 65
[0055] By-product amount (weight%) 2.1 0.70 0.30
[0056] Table 2
Embodiment 4
[0057] Example 4 Example 5 Comparative Example 1
[0058] Feeding molar ratio (formula (1) raw material compound / EpCH) 0.2 0.2 0.2
[0059] Reaction temperature (℃) 120 120 120
[0060] Reaction time (hours) 1.5 1.5 1.5
[0061] Catalyst Type TMAC TMAC TMAC
[0062] Amount added (g) 0.25 0.25 0.25
[0063] Addition rate (weight%) 0.14 0.14 0.14
[0064] Amount of water added (g) 0.20 5.00 0
[0065] Addition rate (weight%) 0.11 2.75 0
[0066] Reaction yield (%) 62 47 62
[0067] By-product amount (weight %) 4.9 0.85 6.4
[0068] As shown in Table 1 and Table 2, under the conditions of the present invention (Examples 1-5), the reaction yield was high, and at least the amount of by-products after vacuum distillation was suppressed. It can be seen that these effects are remarkable when the addition amount of water is adjusted within a specific range (Examples 1 to 3). On the other ha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com