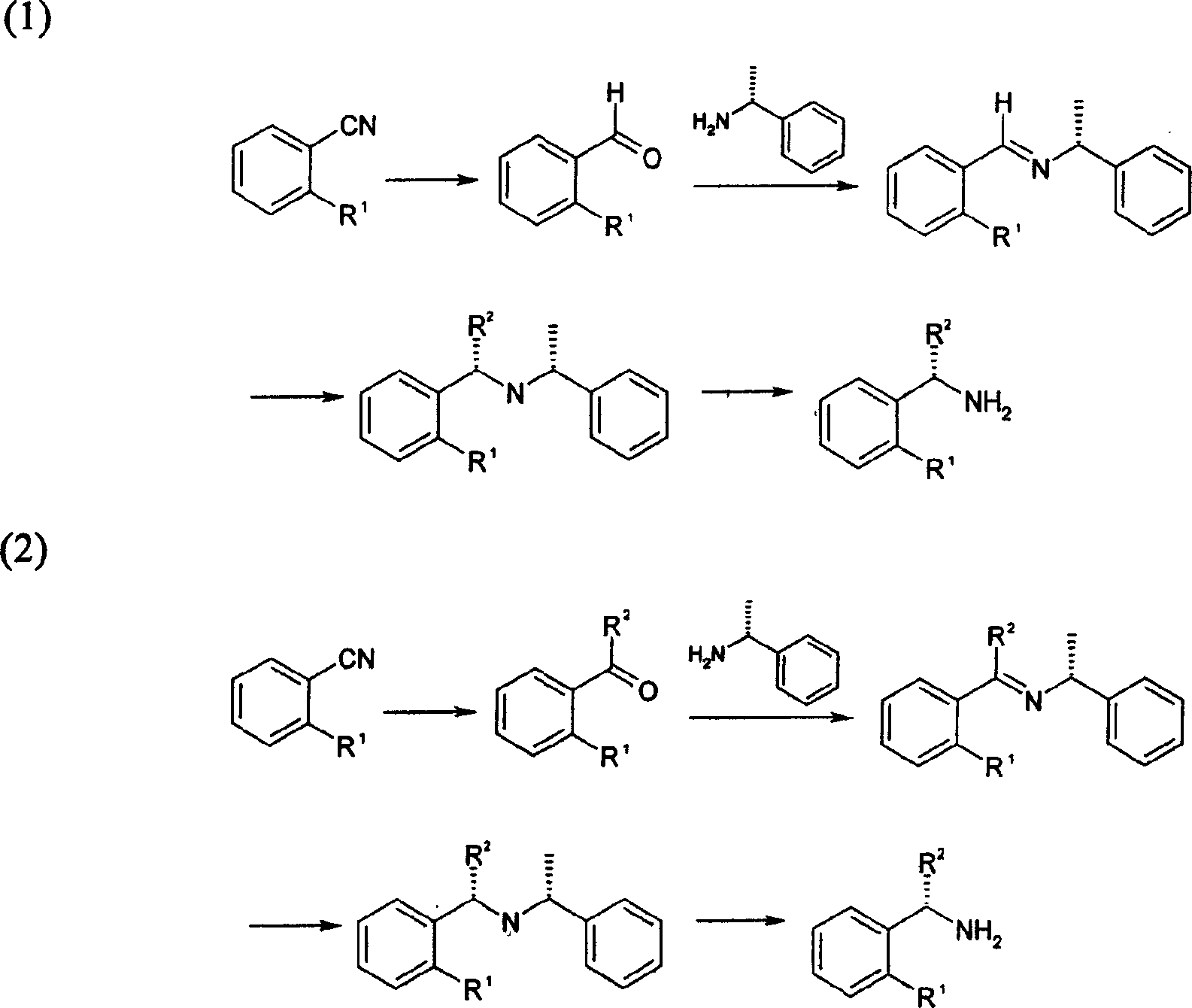
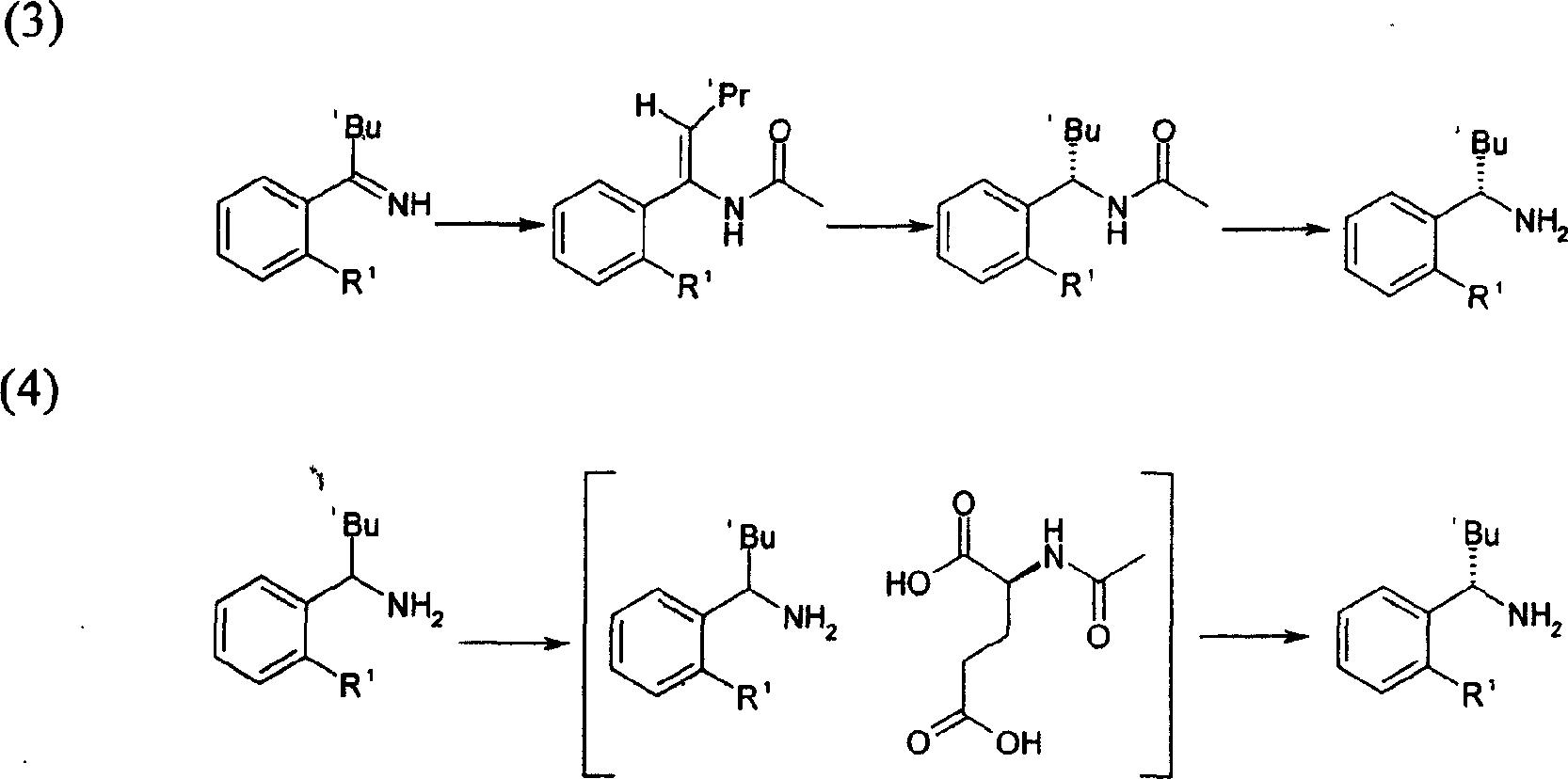
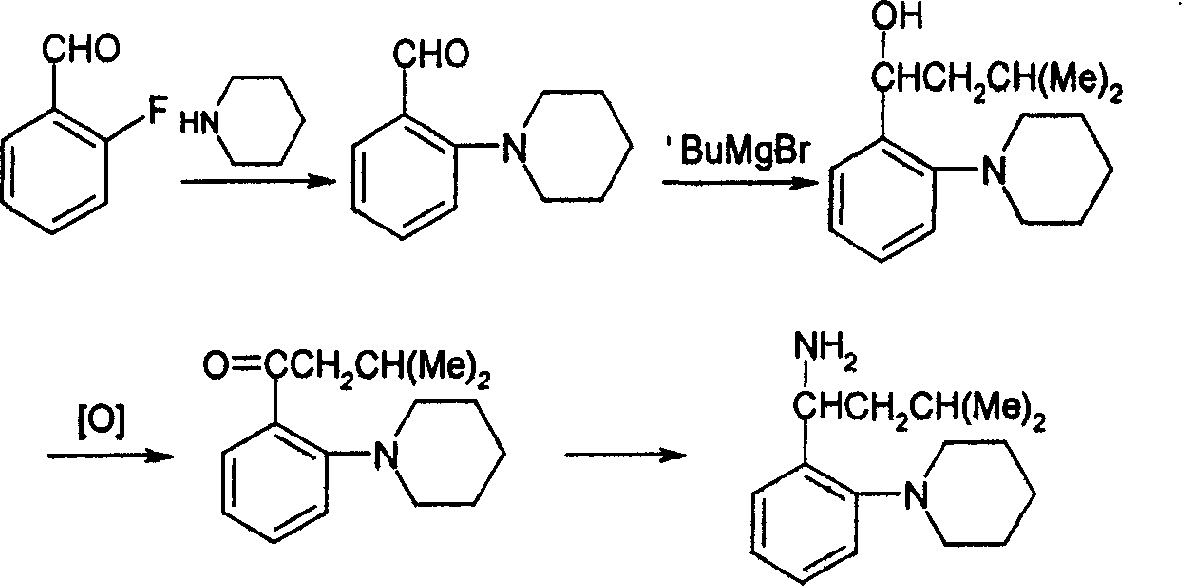
Method for synthesizing (S)-isopropyl-(2-piperidine) phenyl-methylhistamine
A synthesis method and methylamine technology, applied in organic chemistry and other directions, can solve problems such as chiral resolution, and achieve the effects of high optical yield, high reaction yield and simple synthesis route.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Dissolve 20 mmoles of D-(-)-diisopropyl tartrate and 25 moles of trimethylallylborane in 50 ml of anhydrous ether, react at 0°C for 18 hours, concentrate under reduced pressure, and remove by heating The by-product of the reaction was 17 mmoles of D-(-)-methallyl borate isopropyl tartrate ester, and the reaction yield was 85%.
Embodiment 2
[0032] Dissolve 20 mmoles of D-(-)-di-tert-butyl tartrate and 45 moles of trimethylallylborane in 50 ml of anhydrous ether, react at 30°C for 4 hours, concentrate under reduced pressure, and remove by heating The by-product of the reaction was 16.6 mmoles of D-(-)-methallyl borate tert-butyl tartrate ester, and the reaction yield was 83%.
Embodiment 3
[0034] Dissolve 20 millimoles of D-(-)-N, N'-diphenyl tartrate amide and 40 moles of trimethylallyl borane in 50 ml of anhydrous ether, react at 20°C for 10 hours, and then Concentrate under reduced pressure, and heat to remove the reaction by-products to obtain 16.2 mmoles of D-(-)-N, N'-diphenyl-methallyl borate tartrate amide ester, the reaction yield is 81%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com