Doxufylline for injection and its preparing method
A technology for doxofylline and injection, which is applied in the field of pharmaceutical preparations and western medicines, can solve the problems of increased chances of bacterial endotoxin and bacterial contamination, inconvenient compatibility and use of infusion preparations, and contamination of medicinal liquid by glass slag. Accurate and easy-to-control dosage, good formulation appearance, and reduced chance of contamination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0019] Example 1: Formulation Screening - Excipients and their proportions:
[0020] prescription
[0021] preparation
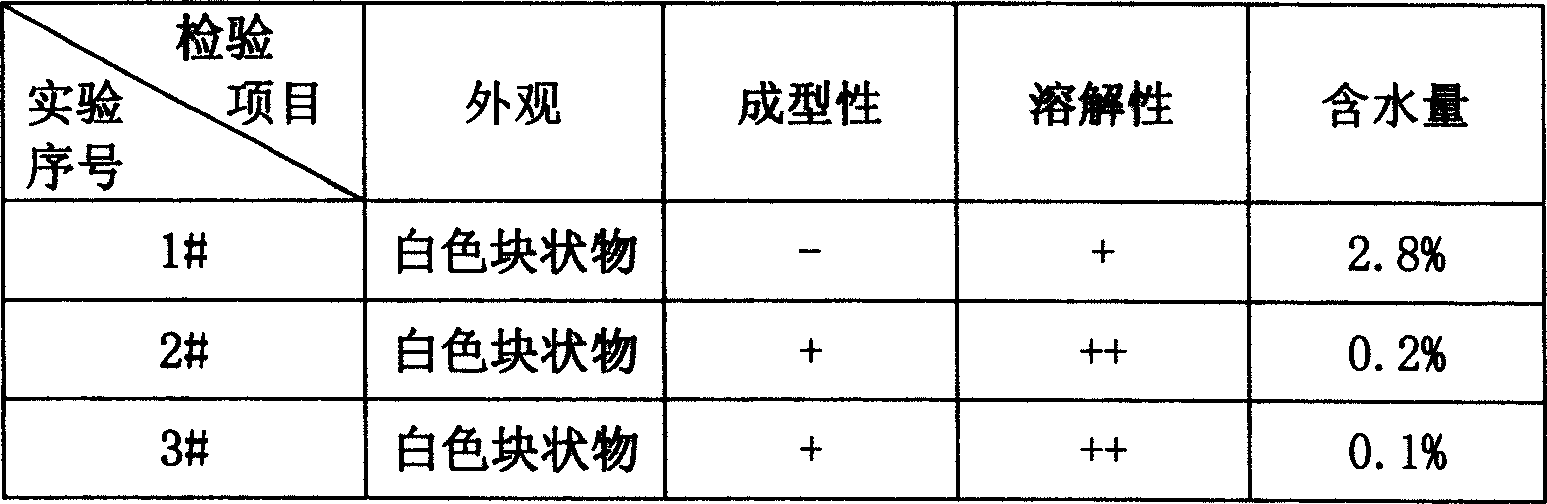
[0022] Dissolve the prescribed amount of excipients in water for injection accounting for 50% of the total amount, add 3‰ needles, heat and boil with activated carbon for 30 minutes, decarbonize and filter, dissolve the prescribed amount of doxofylline in water for injection accounting for 30% of the total amount medium, stir to dissolve, add 3‰ activated carbon for needles, stir for 30 minutes, decarbonize and filter, mix the two filtrates, adjust the pH value to 5.5~6.5 with dilute hydrochloric acid, add water for injection to the total amount—gram weight of doxofylline 50-100 times the number of milliliters, the liquid medicine is filtered with a 0.45 μm microporous membrane, and the sample is taken for semi-finished product content and bacterial endotoxin inspection. 20ml antimicrobial vials, 5ml per bottle, pre-frozen at -35°C for 3 hours, th...
example 2
[0026] Example 2: Investigation of drying process
[0027] Freeze-drying is an optimized process based on the preparation of multiple small samples. It includes three different stages: pre-freezing, decompression freeze-drying, and drying. The three links are an organism, and any improper step will lead to problems in the quality of the finished product, and even the product is unqualified.
[0028] 1) According to the above prescription 9, the eutectic point of the components is -5°C. Therefore, during the decompression freeze-drying process, the temperature should be controlled below -5°C.
[0029] 2) Pre-freezing: Keep at -40°C for 3 hours. If the storage time is too short, the solidification will not be good, and the texture of the sample will be uneven.
[0030] 3) Freeze-drying under reduced pressure: the sample is raised from -35°C to -5°C, and then heated to 0°C, and kept for 19 hours. If the time is too short, the texture of the sample will be affected, making it so...
example 3
[0038] Example 3: Investigation of pH value
[0039] prescription:
[0040] Doxofylline 10.0g
[0041] Mannitol 30.0g
[0042] Water for injection 500ml
[0043] Makes 100
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com