Doxofylline injection
A technology for doxofylline and injection, applied in the field of determination of related substances in injection, can solve the problems of unsolved, no impurity reference substance, difficulty in structure and traceability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
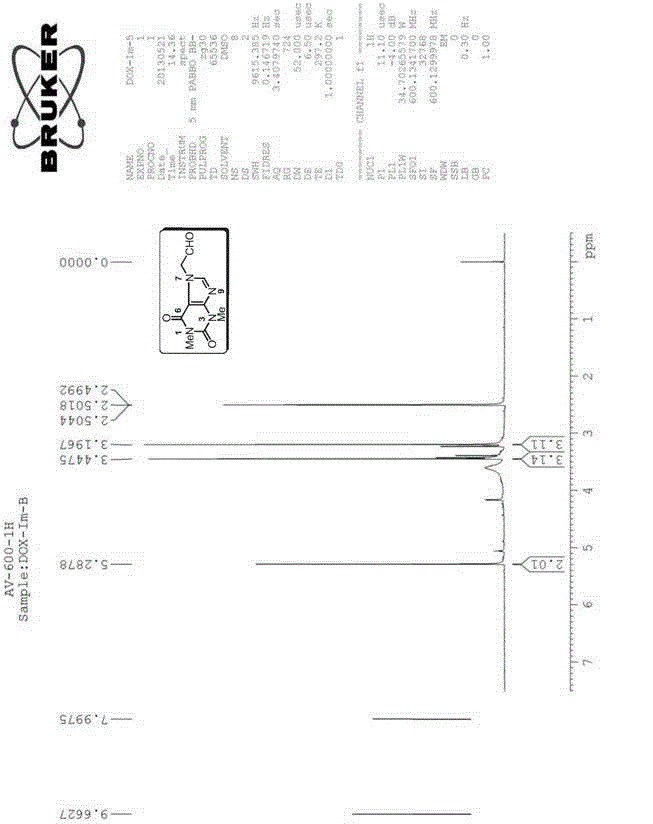
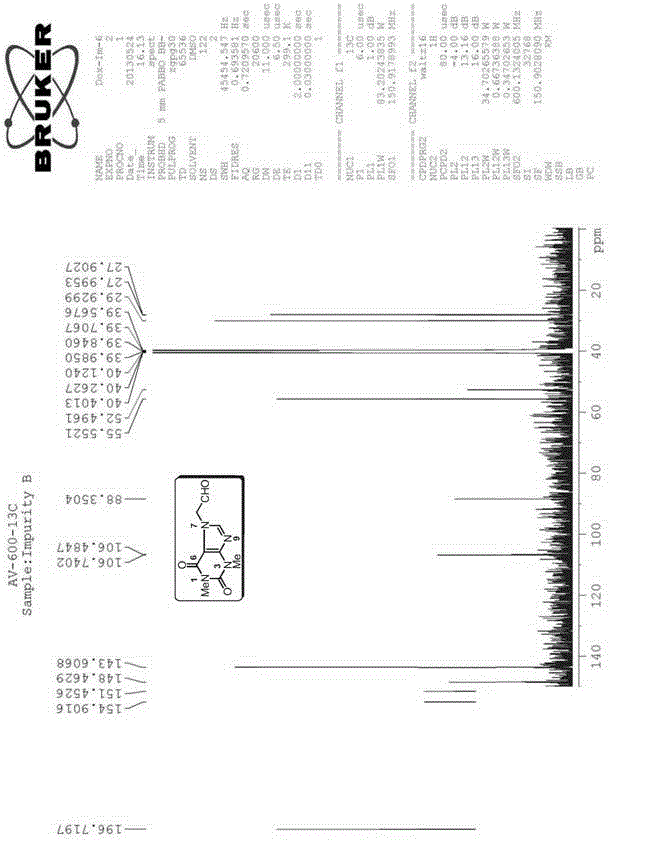
[0154] The preparation of embodiment 1 impurity B (Impurity B) theophylline acetaldehyde:
[0155]
[0156] Preparation process: Add 10.20g (37.50mmol) of doxofylline, 35.0mL of water and 6.0mL of 2.0M hydrochloric acid to a 250mL three-necked bottle in sequence, and gradually heat to reflux, and the reaction solution gradually becomes homogeneous. Continue to reflux for 8h, HPLC monitors that there is no remaining raw material. Concentrate under reduced pressure until about 10 mL of solvent remains, and add 20.0 mL of tetrahydrofuran to disperse to obtain a white solid. Suction filtration, rinse with a small amount of tetrahydrofuran, and dry to obtain 7.81 g of Impurity B, with a yield of 93.8%.
[0157] Structural Confirmation:
[0158] Name: Theophylline acetaldehyde
[0159] Molecular formula: C 9 h 10 N 4 o 3 ;
[0160]Molecular weight: 222.20;
[0161] CAS No: 5614-53-9
[0162] Structural formula:
[0163] Theophylline acetaldehyde purity: HPLC method 9...
Embodiment 2
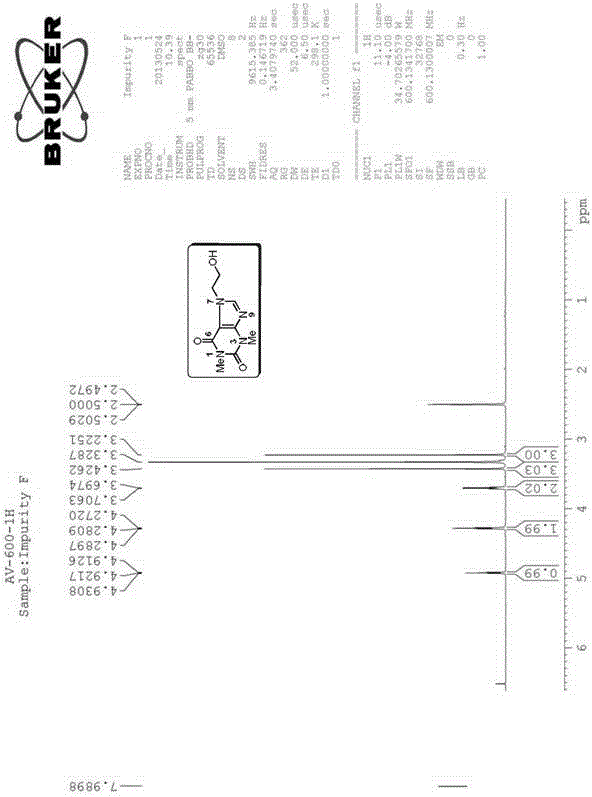
[0183] The preparation of embodiment two impurity F (Impurity F), theophylline ethanol:
[0184] preparation Process: 4.20g (18.0mmol) of Impurity B and 30.0mL of methanol were sequentially added into a 100mL three-necked flask, and 1.11g (29.0mmol) of sodium borohydride was slowly added in an ice-water bath. After the addition was completed, the reaction was gradually raised to room temperature for 2 hours, and no raw materials were detected by HPLC. Adjust the pH to 3-4 with 2.0M hydrochloric acid in an ice-water bath, then adjust the pH to 8-9 with saturated sodium carbonate solution, and distill methanol off under reduced pressure. Extract with water-ethyl acetate, combine the organic layers and dry. Filter and concentrate to obtain a milky white solid. Recrystallize from ethanol, filter, and dry to obtain 2.21 g of Impurity F, with a yield of 55.5%.
[0185] Structural Confirmation:
[0186] Name: Theophylline ethanol
[0187] Molecular formula: C 9 h 12 N 4 o ...
Embodiment 3
[0211] The preparation of embodiment three impurity A (Impurity A):
[0212]
[0213] Preparation process: Add 7.52g (28.15mmol) of doxofylline and 15.0mL of 20% sodium hydroxide aqueous solution to a 250mL three-necked bottle in sequence, heat slowly to 80°C, keep warm for 3 hours, and monitor the end of the reaction by HPLC. Add 50.0 mL of absolute ethanol, cool in an ice-water bath, adjust the pH to 8 with hydrochloric acid, and a large amount of solids are precipitated. Filter and dry to obtain the crude product of Impurity A. After twice recrystallization from ethyl acetate, 5.41 g of Impurity A was obtained, with a yield of 79.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com