Nimustine brain slow release implantation agent and its preparation method
A sustained-release implant, nimustine technology, applied in pharmaceutical formulations, medical preparations containing active ingredients, drug delivery, etc., can solve bone marrow suppression, liver and kidney function poisoning, gastrointestinal reactions, and cross-drug resistance And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
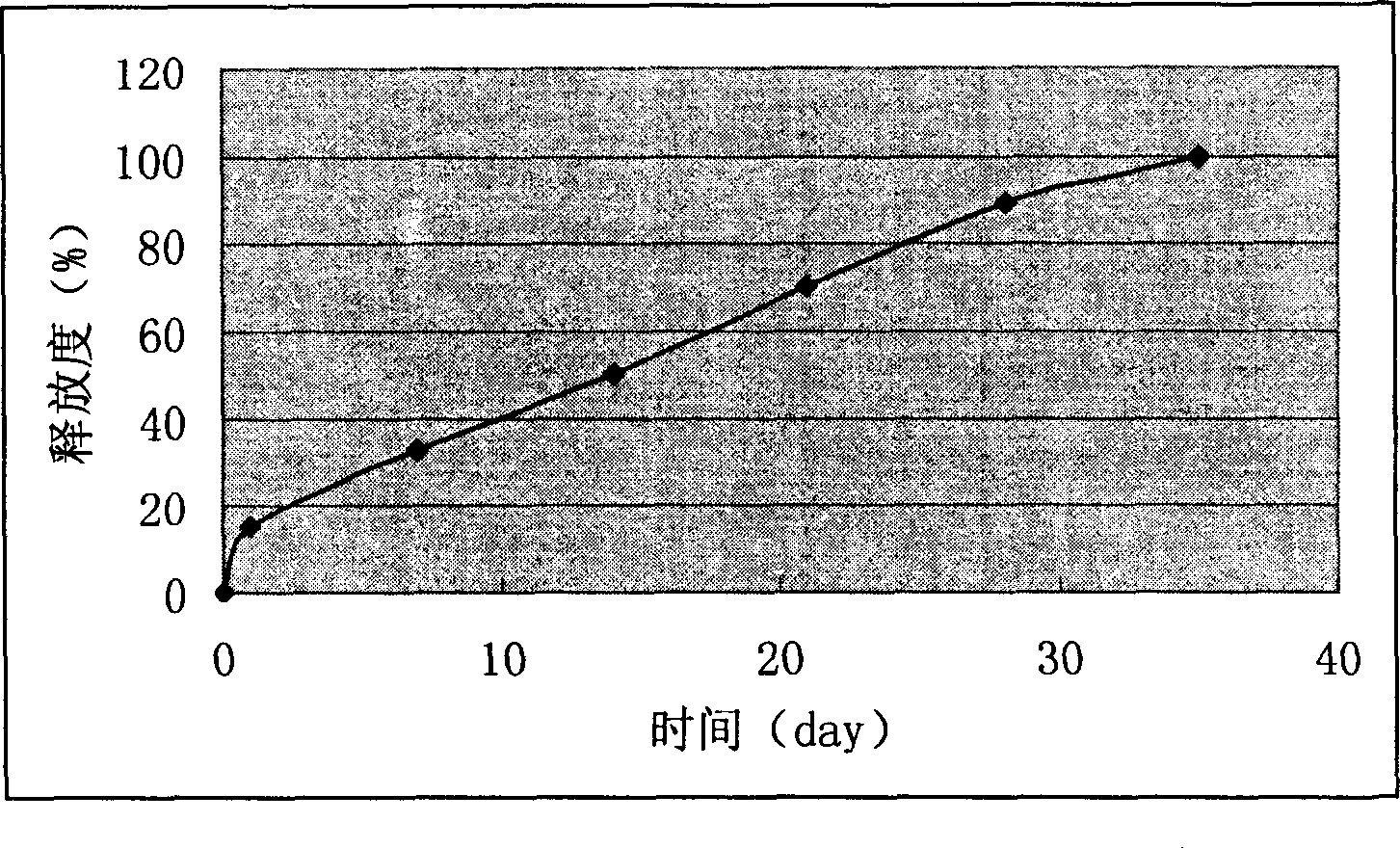
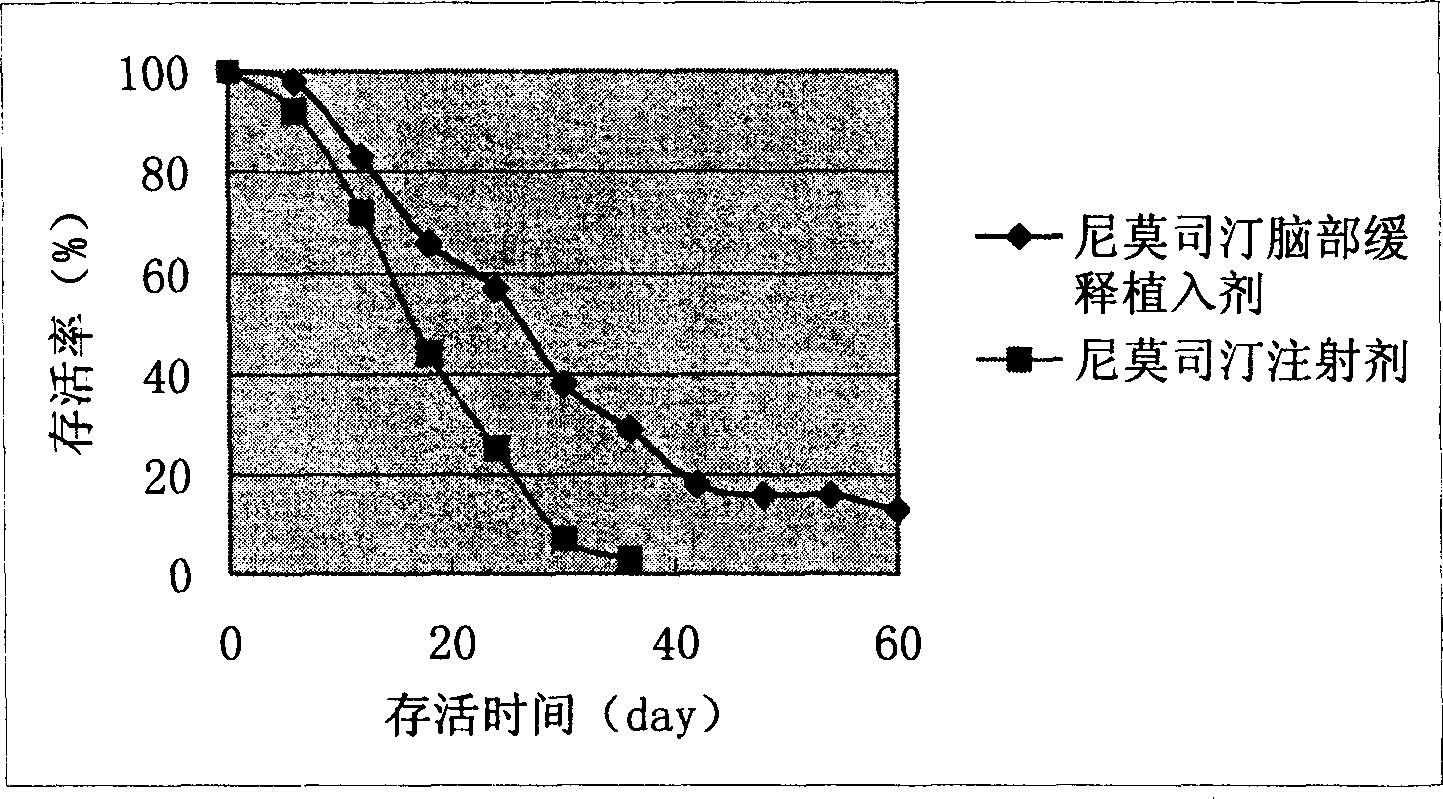
Image
Examples
Embodiment 1
[0034] The weight ratio of nimustine to PLGA is 20:100.
[0035] The sustained-release polymer was a mixture of 60% PLGA (75:25) and dichloromethane solution.
[0036] Preparation steps:
[0037] Using W / O / W double emulsification-solvent evaporation method
[0038] (1) Accurately weigh 20 mg of nimustine into a 1.5 ml plastic centrifuge tube, add 20 μl of 16% gelatin aqueous solution, heat in a water bath at 60° C. to dissolve the drug completely, and serve as the inner water phase.
[0039] (2) Precisely weigh 0.1 g of PLGA and place it in a 1.5 ml plastic centrifuge tube, add 167 μl of dichloromethane to completely dissolve PLGA, and use it as the oil phase.
[0040](3) Add the oil phase to the inner water phase with a pipette gun, vortex mix, heat in a water bath at 60°C, and ultrasonically emulsify to obtain colostrum.
[0041] (4) Cool the colostrum to 18° C. in an ice-water bath, then add it to 40 ml of 4% polyvinyl alcohol 1788 aqueous solution, stir at 8000 rpm for ...
Embodiment 2
[0045] The weight ratio of nimustine to PLGA is 2:100, and the sustained-release polymer is a mixture of 5% PLGA (75:25) and dichloromethane solution. The preparation steps were as in Example 1, and the nimustine brain sustained-release implant was prepared.
Embodiment 3
[0047] The weight ratio of nimustine to PLGA is 5:100, and the slow-release polymer is a mixture of 10% PLGA (75:25) and dichloromethane solution. The preparation steps were as in Example 1, and the nimustine brain sustained-release implant was prepared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com