Cervus and cucumis polypeptide injection composition and preparing method
A deer melon polypeptide and technology for injection, which is applied in the preparation of deer melon polypeptide pharmaceutical composition for injection, and in the field of deer melon polypeptide pharmaceutical composition for injection, can solve problems such as inconsistency, and achieve the goal of inhibiting release, promoting recovery, and promoting synthesis Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
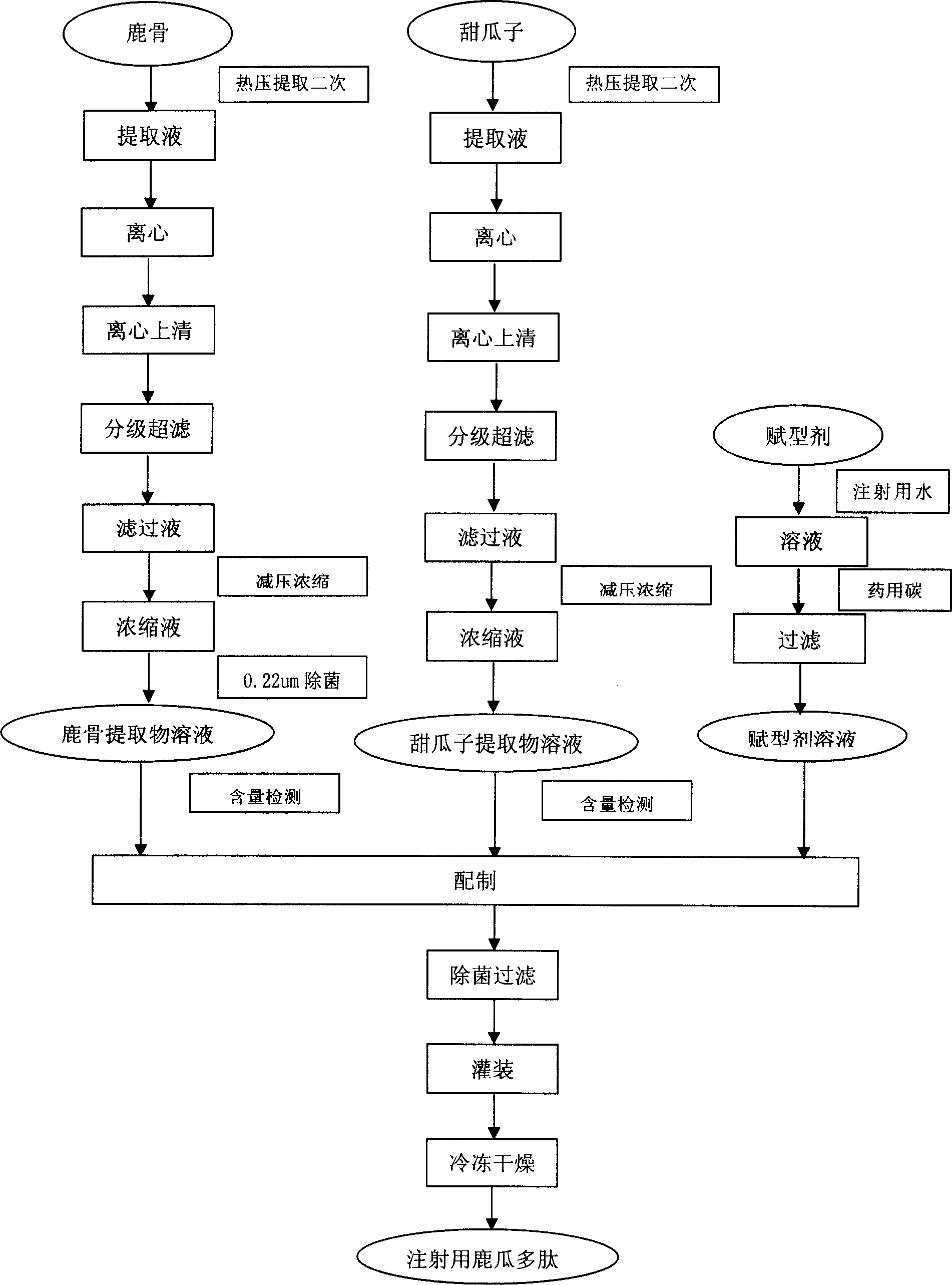
[0085] Embodiment 1: the preparation of deer bone extract solution
[0086] Boil the deer bones in boiling water for 30 minutes, take them out, remove the fascia and residual meat, and obtain the deer bones. Take the deer bone and crush it to a size less than 1cm for later use. Take 30kg of broken bones, add 60kg of water for injection, extract by hot pressing at 121°C for 2 hours, and filter out the primary extract; then add 60kg of water for injection, extract by hot pressing at 121°C for 2 hours, filter out the second extract, combine the filtrates, and let them stand overnight. Take the deer bone extract, centrifuge (4000 rpm, 15 minutes), take the supernatant, and discard the precipitate. Take the supernatant, and carry out graded ultrafiltration, 100KD, 10KD, 6KD, take the filtrate each time, and discard the concentrate. Take the filtrate, concentrate under reduced pressure to 10% of the total volume, collect the concentrated solution, adjust the pH value to 6.0-6.5, ...
Embodiment 2
[0088] Embodiment 2: the preparation of muskmelon seed extract solution
[0089]Take the melon seeds, put them in water for injection, rinse repeatedly 3-4 times, dry the water, pick out the sundries, and get the clean melon seeds for later use. Take 15kg of clean melon seeds, add 30kg of water for injection, and grind them into a homogenate of melon seeds with a colloid mill. Take melon seeds homogenate, extract by hot pressing at 121°C for 2 hours, and filter out the primary extract; then add 30kg of water for injection, continue to extract by hot pressing at 121°C for 2 hours, filter out the second extract, combine the filtrates, and leave overnight. Take the melon seed extract, centrifuge (4000 rpm, 15 minutes), take the supernatant, and discard the precipitate. Take the supernatant, and carry out graded ultrafiltration: 100KD, 10KD, 6KD, take the filtrate each time, and discard the concentrate. Take the final filtrate, concentrate under reduced pressure to 10% of the ...
Embodiment 3
[0091] Embodiment 3: pharmaceutical composition 1
[0092] Preparation prescription:
[0093] The deer bone extract solution 26.7g of embodiment 1 (calculated by deer bone polypeptide)
[0094] Melon seed extract solution 13.3g (calculated by the melon seed polypeptide) of embodiment 2
[0095] 20% excipient solution 5000ml
[0096] 5% NaOH (or 5% HCl) appropriate amount
[0097] Add water for injection to 20000ml
[0098] The deer bone extract solution prepared in Example 1 and the melon seed extract solution prepared in Example 2 were mixed according to the weight ratio of deer bone polypeptide and melon seed polypeptide in a ratio of 2:1, and 5000ml of excipient (dextran 40, glucose or lactose) solution was added , adjust the pH value of the solution to 5.5-7.0 with an appropriate amount of 5% NaOH or 5% HCl, then add water for injection to the full amount, and make a 4 mg preparation by filling, freeze-drying, capping and other steps.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com