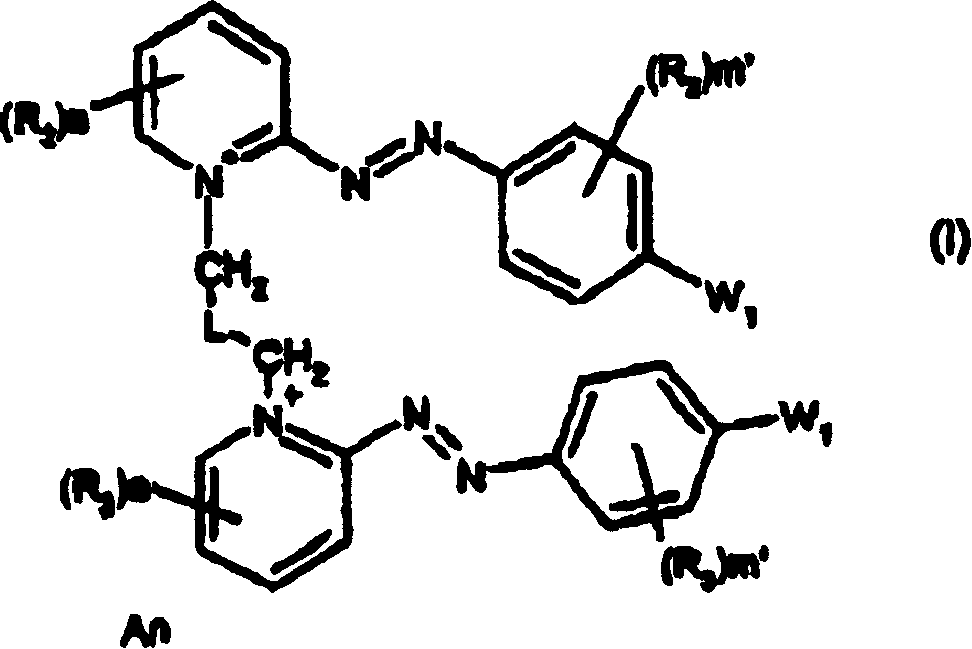
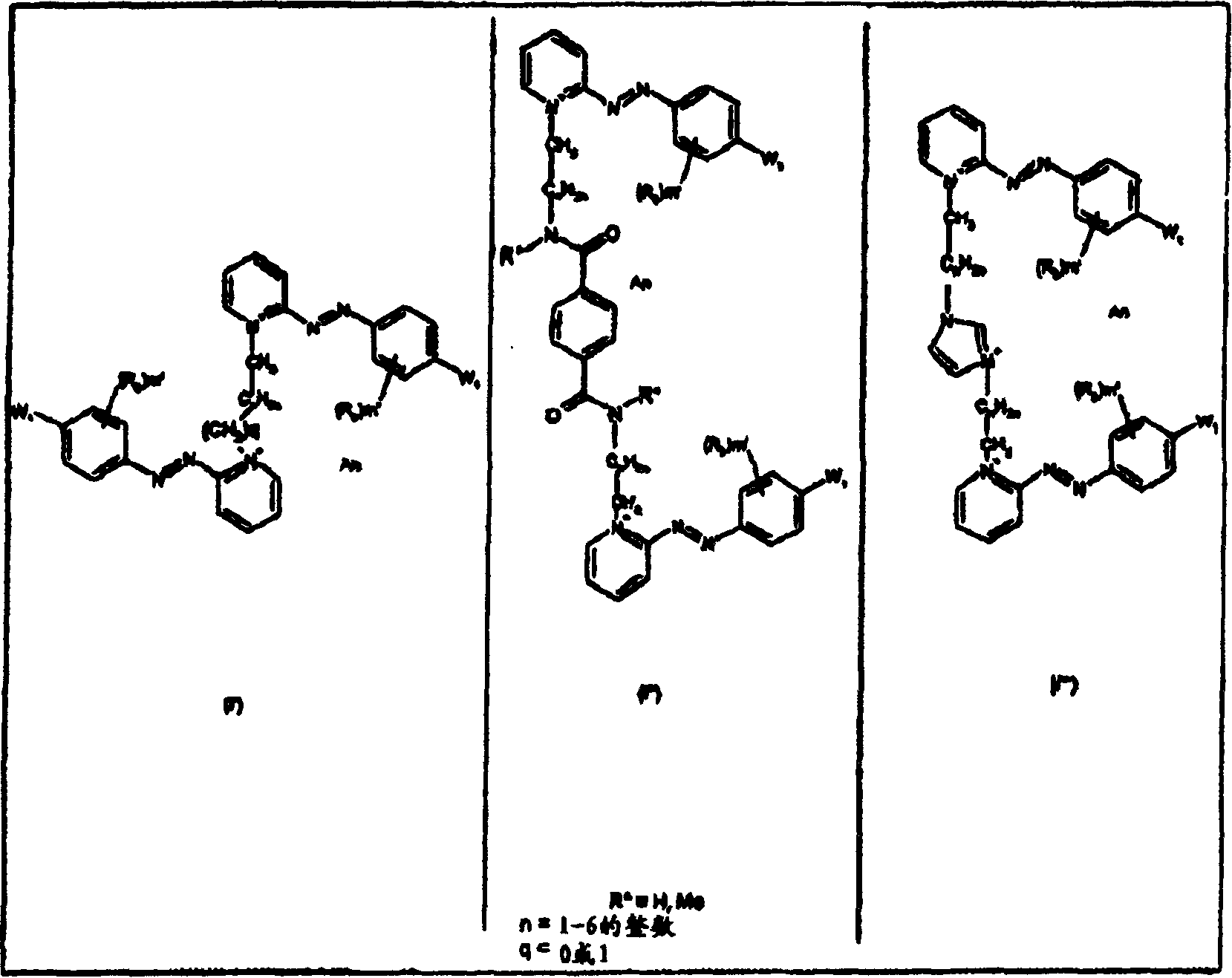
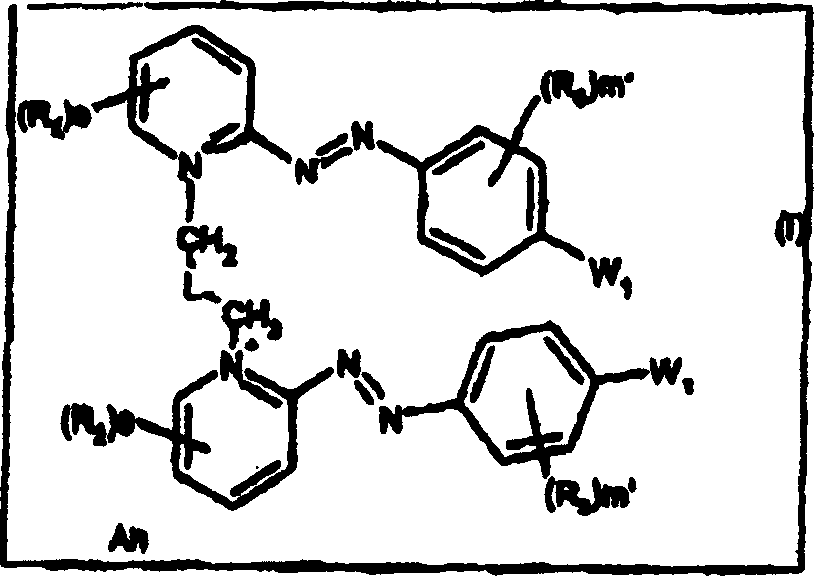
Dissymmetrical diazo compounds comprising 2-pyridinium group and a cationic or non-cationic linker, compositions comprising them, method for coloring, and device
A diazo compound, cationic technology, applied in the field of devices with multiple compartments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0409] Synthesis of Compound 4 : 5-bromo-2-{(E)-[4-(dimethylamino)-2-hydroxyphenyl]diazenyl}-1-[3-(2-{(E)-[4- Synthesis of (Dimethylamino)phenyl]diazenyl}pyridinium-1-yl)propyl]pyridinium dibromide
[0410]
[0411] compound 4
[0412] step 1
[0413]
[0414] Compound 1 is a commercially available compound.
[0415] Compound 1 (30 g) was stirred in 350 ml toluene at 100° C. for 14 hours in the presence of 68 ml 1,3-dibromopropane in a three-necked flask with a fixed top condenser.
[0416] After the reaction, the reaction mixture was cooled to ambient temperature and poured into ethyl acetate (500 mL). The resulting precipitate was isolated by filtration, washed several times with ethyl acetate and finally dried in vacuo. 44 mg of dark purple powder corresponding to compound 2 were obtained.
[0417] Analyze according to expected product.
[0418] step 2
[0419]
[0420] Compound 3 is a commercially available product.
[0421] In a three-necked flask wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com