Enol form lactide open-ring polymerization catalyst and its preparing method and use
A technology of ring-opening polymerization and lactide, applied in the field of enol lactide ring-opening polymerization catalyst and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
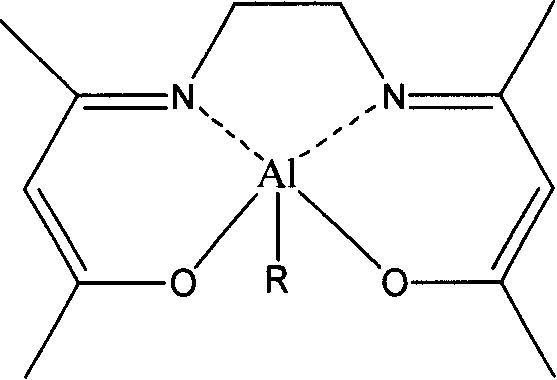
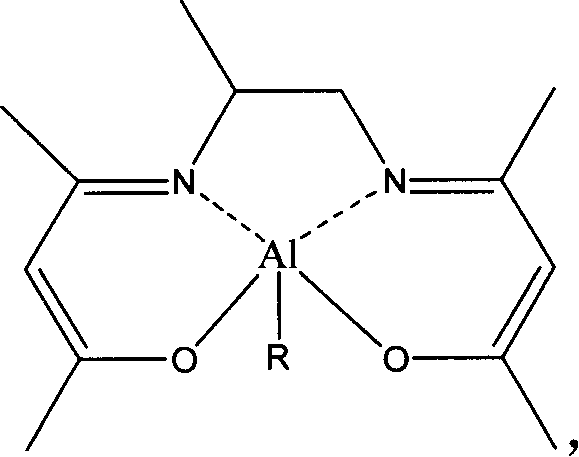
[0046] Embodiment 1: structural formula is the synthesis of the 1a catalyst of I
[0047] Under the protection condition of inert gas, the concentration is 1mol / L 4ml the toluene solution of the Schiff base of structural formula 1, and the concentration is 1mol / L 4ml AlEt 3The toluene solutions were mixed, put in an oil bath at 90° C. and stirred, reacted for 10 hours, then cooled to room temperature, and evacuated to remove volatile substances. The vacuum degree was 0.1 MPa to obtain the 1a catalyst with product structural formula I. Elemental analysis results, calculated value: C, 60.41; H, 8.33; N, 10.06; measured value: C, 61.05; H, 8.67; N, 10.51.
Embodiment 2
[0048] Embodiment 2: structural formula is the synthesis of the 1e catalyst of 1b of I, 1c of I, 1d of I or I
[0049] The enol type lactide ring-opening polymerization catalyst (557mg, 2mmol) of 1a that the structural formula that embodiment 1 obtains is dissolved in toluene, and to the toluene solution that obtains, add the methyl alcohol with the solute of this kind of toluene solution etc. , the structural formula of the present invention can be obtained as the 1b catalyst of I.
[0050] If the methanol in this embodiment is replaced by ethanol, isopropanol or benzyl alcohol respectively, and other conditions are the same, the 1c of catalyst I, the 1d of I or the 1e of I can be obtained respectively.
[0051] 1b elemental analysis results of I, calculated value: C, 55.70; H, 7.55; N, 9.99; found value: C, 55.93; H, 7.28; N, 10.20.
[0052] The elemental analysis results of 1c of I, calculated value: C, 57.13; H, 7.88; N, 9.52; found value: C, 57.35; H, 7.62; N, 9.81.
[...
Embodiment 3
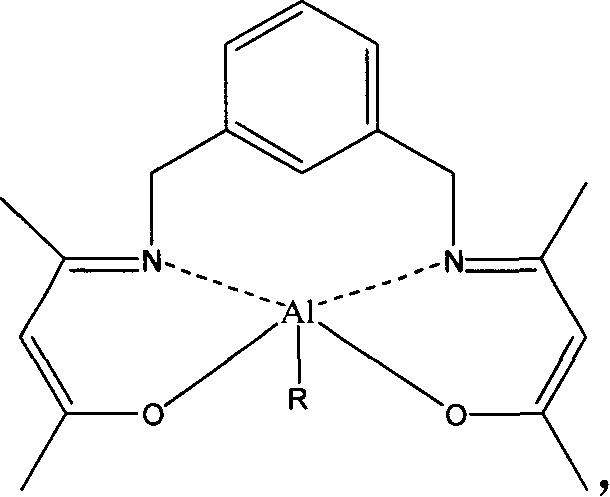
[0055] Embodiment 3: structural formula is the synthesis of 2a catalyst of II
[0056] Replace Schiff base 1 in Example 1 with Schiff base 2, and other conditions are the same as in Example 1 to obtain 2a of product II. Elemental analysis results, calculated value: C, 61.62; H, 8.62; N, 9.58; measured value: C, 60.82; H, 8.40; N, 10.06.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com