Quadridentate pyridyl schiff base metal complex and preparation method thereof as well as preparation method of cyclic carbonate
A technology of tetradentate pyridine-based mats and metal complexes, applied in organic compound/hydride/coordination complex catalysts, magnesium organic compounds, zinc organic compounds, etc., can solve the problems of low selectivity, long reaction time, and catalytic activity. Low-level problems, to achieve the effect of promoting the application and preventing the content of toxic metals from exceeding the standard
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
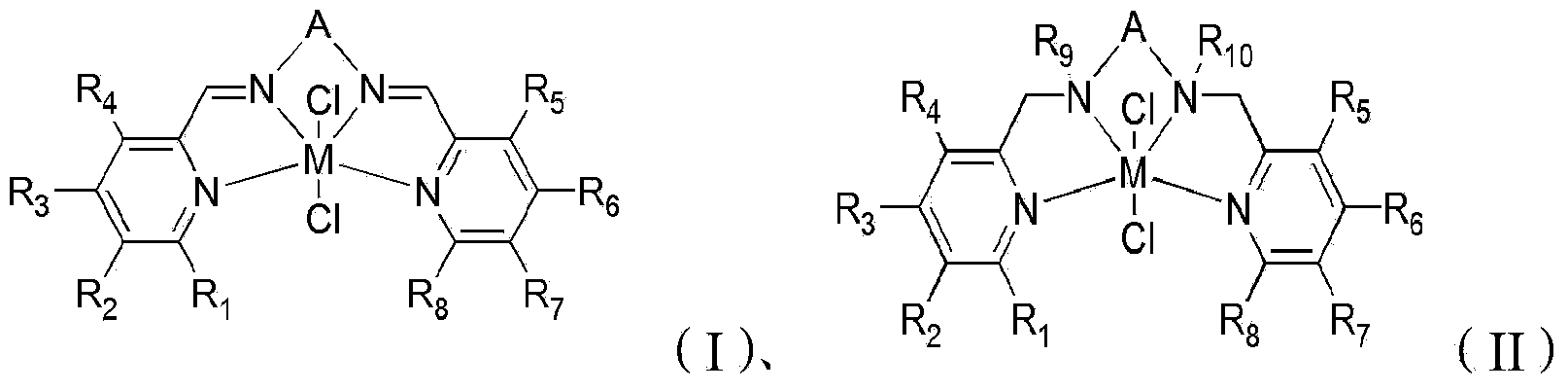
[0101] The present invention also provides a preparation method of a tetradentate pyridyl Schiff base metal complex having a structure represented by formula (II), comprising the following steps:
[0102] reacting the first intermediate product obtained by the above technical scheme with a reducing agent in a solvent to obtain a second intermediate product;
[0103] The second intermediate product is reacted with a metal chloride in an organic solvent to obtain a tetradentate pyridyl Schiff base metal complex having a structure represented by formula (II).
[0104] In the present invention, the first intermediate product is preferably dissolved, and then the obtained first intermediate product solution is cooled, and then a reducing agent is added therein, stirred, and reacted to obtain a second intermediate product. In the present invention, the cooling temperature is preferably -5°C to 5°C, more preferably -2°C to 2°C, most preferably 0°C.
[0105] In the present invention,...
Embodiment 1
[0127] Add 5.63g of 2-pyridinecarbaldehyde and 3.00g of 1,2-cyclohexanediamine into 50mL of anhydrous methanol, stir and react the resulting mixed solution at room temperature for 4h, remove the solvent in the reaction solution in vacuo, and dissolve the obtained solid with diethyl ether The reaction product was obtained after washing.
[0128] The present invention carried out structural identification of the obtained reaction product, and the results showed that the reaction product obtained in this example had the structure shown in formula (XX);
[0129] The present invention calculates that the yield of the compound represented by the formula (XX) in this example is 75%.
[0130]
[0131] 2.00 g of the compound represented by the formula (XX) was dissolved in dichloromethane, and an excess of ferric chloride was added thereto. The reaction was stirred overnight at room temperature and filtered. Diethyl ether was added to produce precipitation, and the tetradentate py...
Embodiment 2
[0134] Dissolve 2.00 g of the compound of the formula (XX) obtained in Example 1 in 25 mL of anhydrous methanol, cool the obtained solution of the compound of the formula (XX) to 0°C, and slowly add 2.59g NaBH 4 , the reaction system was warmed up to room temperature and continued to stir for 12 h. Remove the solvent in the reaction solution in vacuo, add 100mL dichloromethane and 50mL water to the obtained solid for extraction, collect the organic phase; extract the water phase with 100mL dichloromethane for 2 times, combine the organic phases with anhydrous magnesium sulfate Drying, filtration to remove magnesium sulfate, and removal of solvent in vacuo afforded the reaction product.
[0135] The present invention carried out structural identification of the obtained reaction product, and the results showed that the reaction product obtained in this example had the structure shown in formula (XXI);
[0136] The present invention calculates that the yield of the compound ha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com