A kind of preparation method of cyclic carbonate
A technology of cyclic carbonate and cyclization reaction, which is applied in the direction of chemical instruments and methods, organic compound/hydride/coordination complex catalyst, catalytic reaction, etc., can solve the problem of no cyclic carbonate, etc., to prevent poisonous The metal content exceeds the standard, which is conducive to the effect of popularization and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The invention provides a kind of preparation method of cyclic carbonate, comprises the following steps:
[0030] Under the action of the main catalyst and the co-catalyst, the carbon dioxide and the epoxide are subjected to a cyclization reaction to obtain a cyclic carbonate;
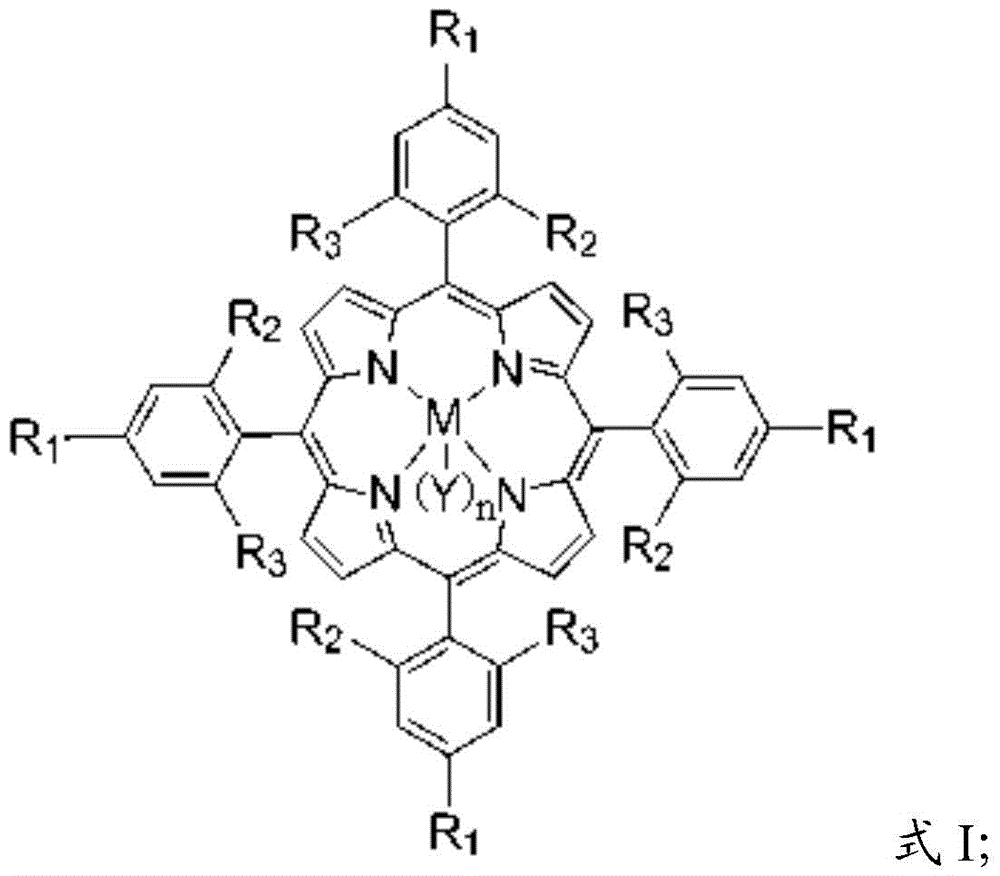
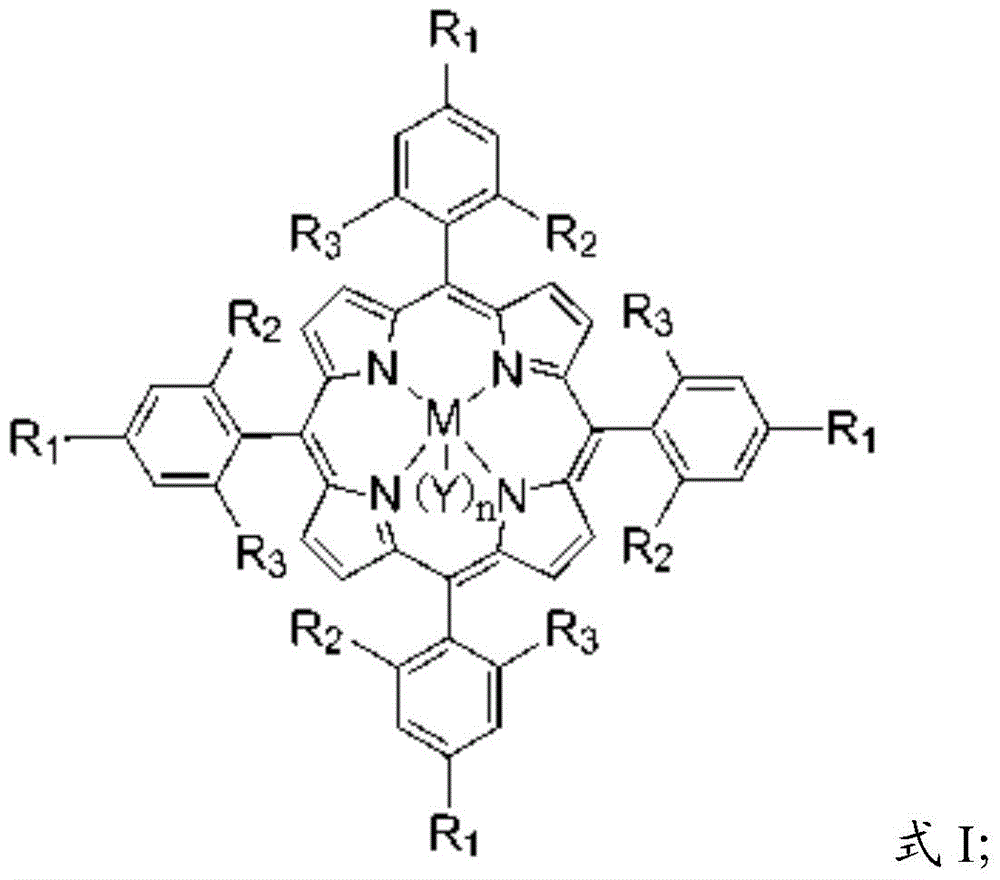
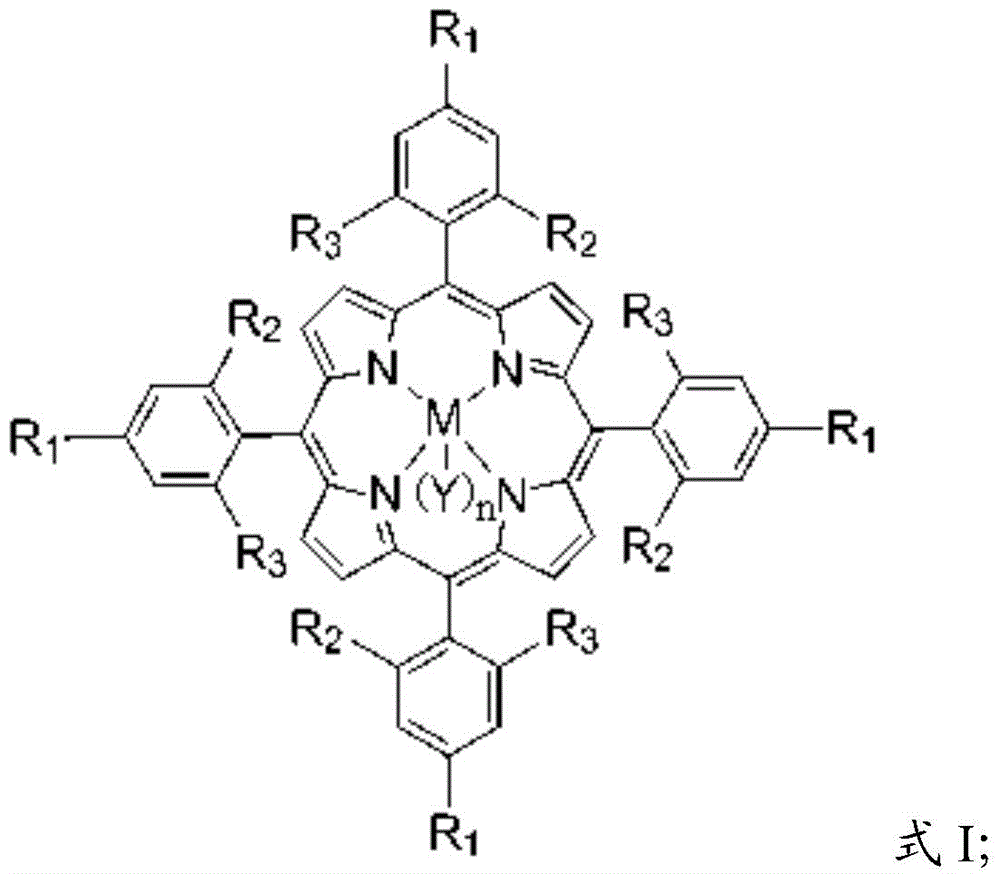
[0031] The main catalyst is a metal porphyrin complex, and the metal porphyrin complex has a structure shown in formula I:
[0032]
[0033] In formula I, the R 1 , R 2 and R 3 independently selected from hydrogen, halogen, aliphatic, substituted aliphatic, substituted heteroaliphatic, aryl, substituted aryl, or substituted heteroaryl;
[0034] The M is a metal element;
[0035] n=0 or 1;
[0036] The Y is a halogen group, -NO 3 、CH 3 COO-, CCl 3 COO-, CF 3 COO-, ClO 4 -, BF 4 -, BPh 4 -, -CN, -N 3 , p-toluate, p-toluenesulfonate, o-nitrophenol oxyanion, p-nitrophenol oxyanion, m-nitrophenol oxyanion, 2,4-dinitrophenol oxyanion, 3,5 -Dinitrophenol oxyanion, 2,4,6-trinitrophenol o...
Embodiment 1
[0111] at 25°C, N 2 Under protection, add 800mL dry dichloromethane, 2.8g 4-chlorobenzaldehyde (20mmol), 1.4mL pyrrole (20mmol) into a 1000mL round-bottomed three-neck flask, stir until the solid is completely dissolved, then add 3.7mL trifluoroacetic acid (50mmol, 2.5eq) and stirred for 1h, then added 9.08g DDQ (2.3-dichloro-5,6-dicyano-1,4-benzoquinone) and stirred for 1h, filtered the above liquid under reduced pressure, and distilled off the solvent under reduced pressure , the resulting crude product is separated by chromatographic column [stationary phase: Al2O3; mobile phase: methylene chloride / petroleum ether (volume ratio)=2:1], to obtain four (4-chlorophenyl) porphyrin, product conversion Rate 20.8%; The present invention carries out proton nuclear magnetic resonance spectrum test to the tetrakis (4-chlorophenyl) porphyrin that obtains, and analysis result is: 1 H NMR (300MHz, CDCl 3 ), δ: 8.83(s, 8H), 8.12(d, J=9.0Hz, 8H), 7.78(d, J=9.0Hz, 8H), -2.87(s, 2H); (4-c...
Embodiment 2
[0117] at 25°C, N 2 Under protection, add 800mL of dry dichloromethane, 3.7g of 4-bromobenzaldehyde (20mmol), and 1.4mL of pyrrole (20mmol) into a 1000mL round-bottomed three-neck flask, stir until the solid is completely dissolved, then add 3.7mL of trifluoroacetic acid ( 50mmol, 2.5eq) and stirred for 1h, then added 9.08g DDQ (2.3-dichloro-5,6-dicyano-1,4-benzoquinone) and stirred for 1h. The above liquid was filtered under reduced pressure, the solvent was distilled off under reduced pressure, and the obtained crude product was separated by chromatographic column [stationary phase: aluminum oxide; mobile phase: dichloromethane / petroleum ether (volume ratio)=2:1] to obtain four (4-Bromophenyl) porphyrin, the product conversion rate is 19.5%. 1 HNMR (300MHz, CDCl 3 ), δ: 8.84(s, 8H), 8.06(d, J=9.0Hz, 8H), 7.91(d, J=9.0Hz, 8H), -2.86(s, 2H); (4-bromophenyl) porphyrin has structure shown in formula XIV:
[0118]
[0119] at 25°C, N 2 Add 20mL of dry dichloromethane and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com