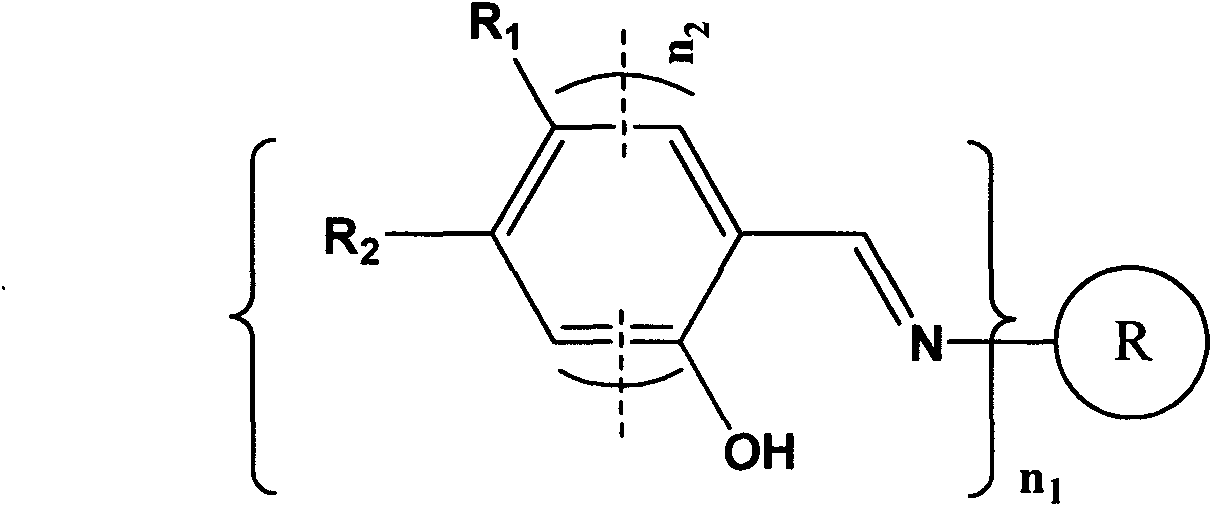
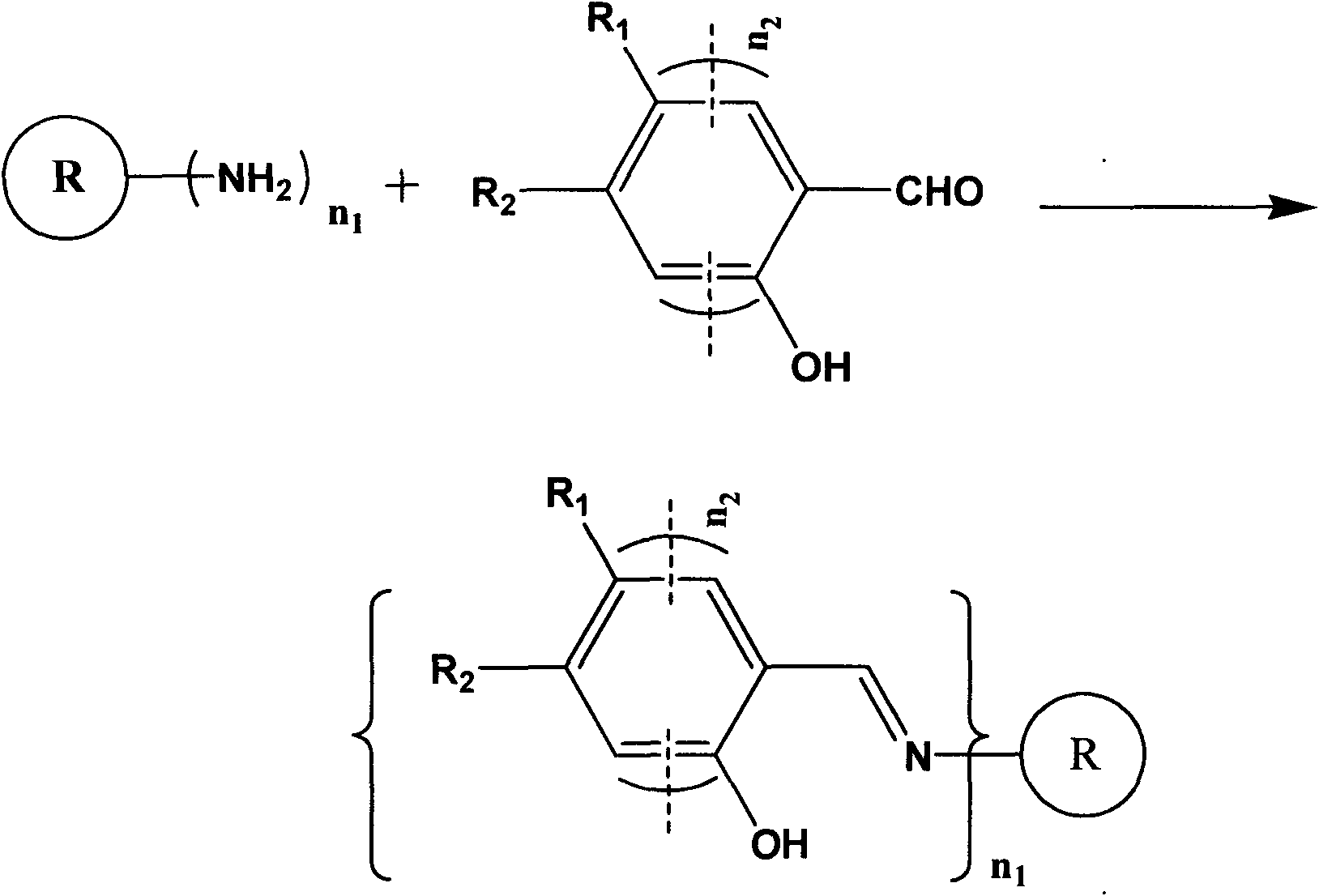
Schiff base compound and method for detecting aniline compound thereof
An aniline compound and detection method technology are applied in the field of preparation of organic fluorescent sensor materials, which can solve problems such as limited sensing performance, and achieve the effects of high luminous efficiency, structural adjustment, and strong chemical modification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The synthetic route of Schiff base compound is as follows figure 2 shown
[0041] (1) Synthesis of N, N'-disalicylaldehyde azine (n1=2, n2=1, R1=R2=H)
[0042]
[0043] Weigh 1.20g of salicylaldehyde and dissolve it in absolute ethanol, heat it in a water bath to 80°C, slowly add 0.16g of 85wt% hydrazine hydrate dropwise, and reflux for 1.5h to obtain a yellow precipitate. After recrystallization, it was filtered and dried to obtain the target compound 1 as a bright yellow solid with a mass of 1.20 g (yield 88.6%).
[0044] Mass spectrum (EI): m / z240
[0045] Melting point: 219°C
[0046] H NMR 1 H-NMR (400MHz, CDCl 3 , 25° C., TMS): δ=11.12 (s, 2H), 9.00 (s, 2H), 7.71-7.68 (m, 2H), 7.41 (m, 2H), 6.97 (m, 4H). Elemental Analysis C 14 h 12 N 2 o 2 (240.09) Calculated: C, 69.99; H, 5.03; N, 11.66. Measured values: C, 70.05; H, 5.04; N, 11.61.
[0047] (2) N, the synthesis of N'-two 2-nitrosalicylaldehyde condensed nitrogen (n 1 = 2, n 2 = 1, R 1 =-NO 2...
Embodiment 2
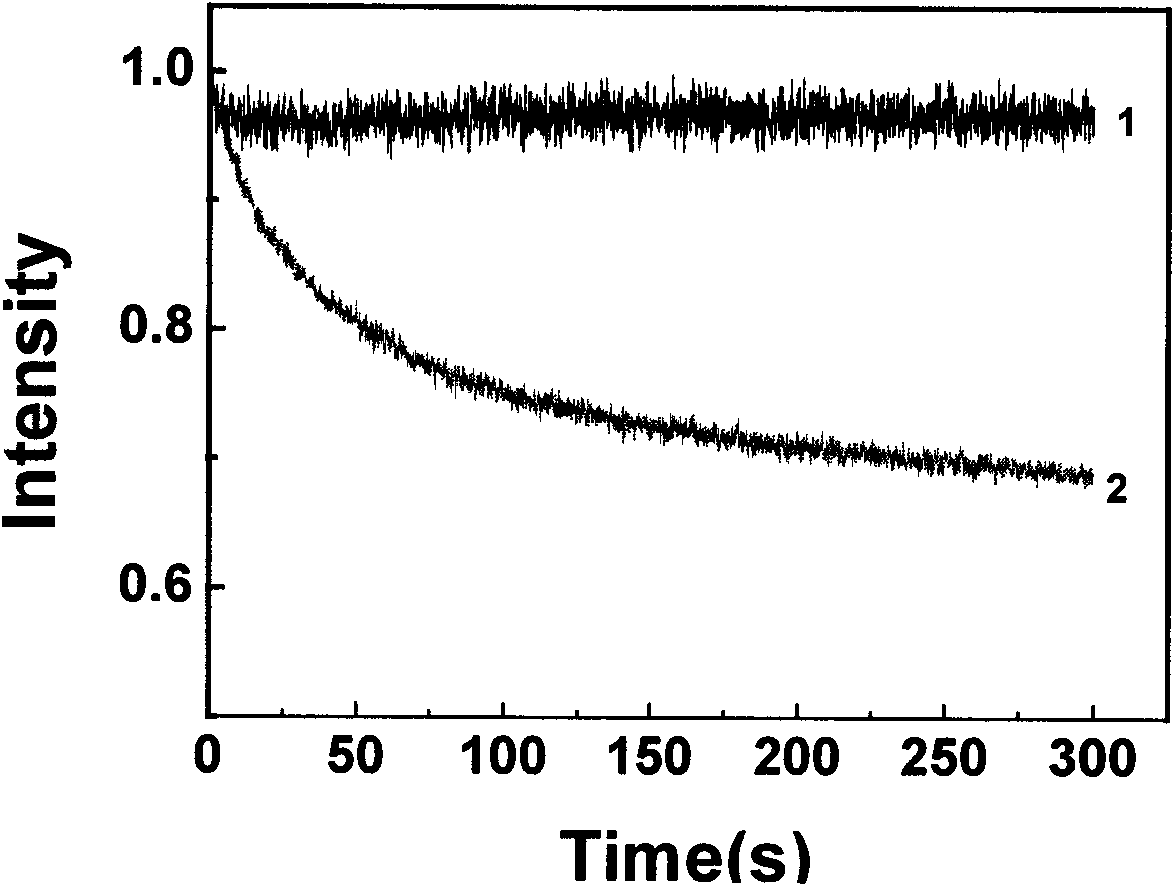
[0110] A sensing film based on N,N'-disalicylaldehyde azide was prepared on a quartz wafer substrate (ie, the support, the same below) by a pulling method. Test the fluorescence spectrum and photostability of the sensing film. Drop a drop of aniline on the bottom of the quartz cell, place a ball of absorbent cotton above it to avoid direct contact with the sensing film, and seal the quartz cell with a cover. After placing the sensing film in a closed quartz cell, quickly measure the curve of the peak intensity at the maximum fluorescence emission peak with time. Such as image 3 shown.
Embodiment 3
[0112] A sensing film based on p-phenylenediamine disalicylaldehyde was prepared on a quartz substrate by pulling method. Test the fluorescence spectrum and photostability of the sensing film. Drop a drop of aniline on the bottom of the quartz cell, place a ball of absorbent cotton above it to avoid direct contact with the sensing film, and seal the quartz cell with a cover. After placing the sensing film in a closed quartz cell, quickly measure the curve of the peak intensity at the maximum fluorescence emission peak with time. Such as Figure 4 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com