Polypeptides having binding affinity for HER2
A technology of affinity and structural domain, applied in the field of new peptides, can solve problems such as bone marrow suppression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
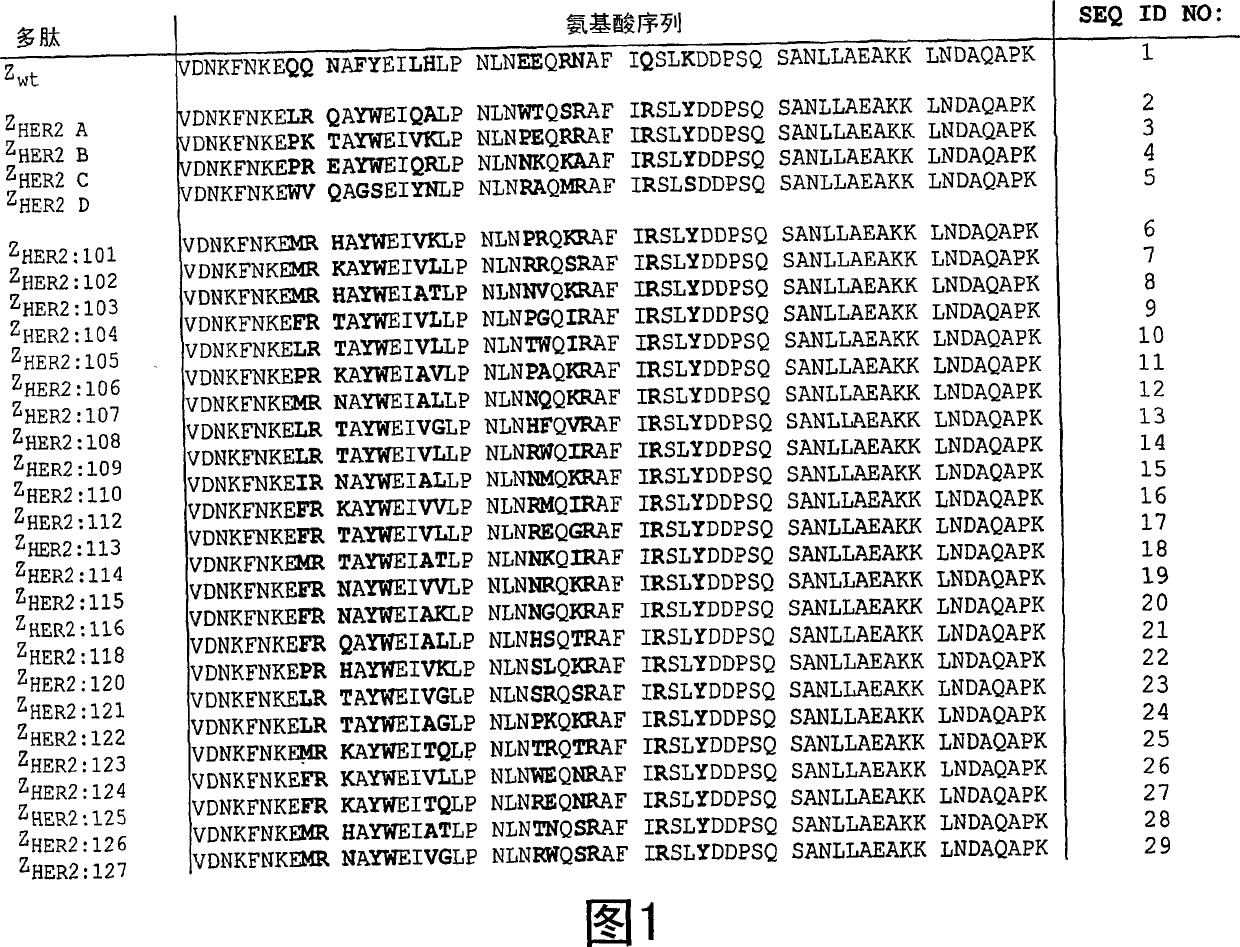
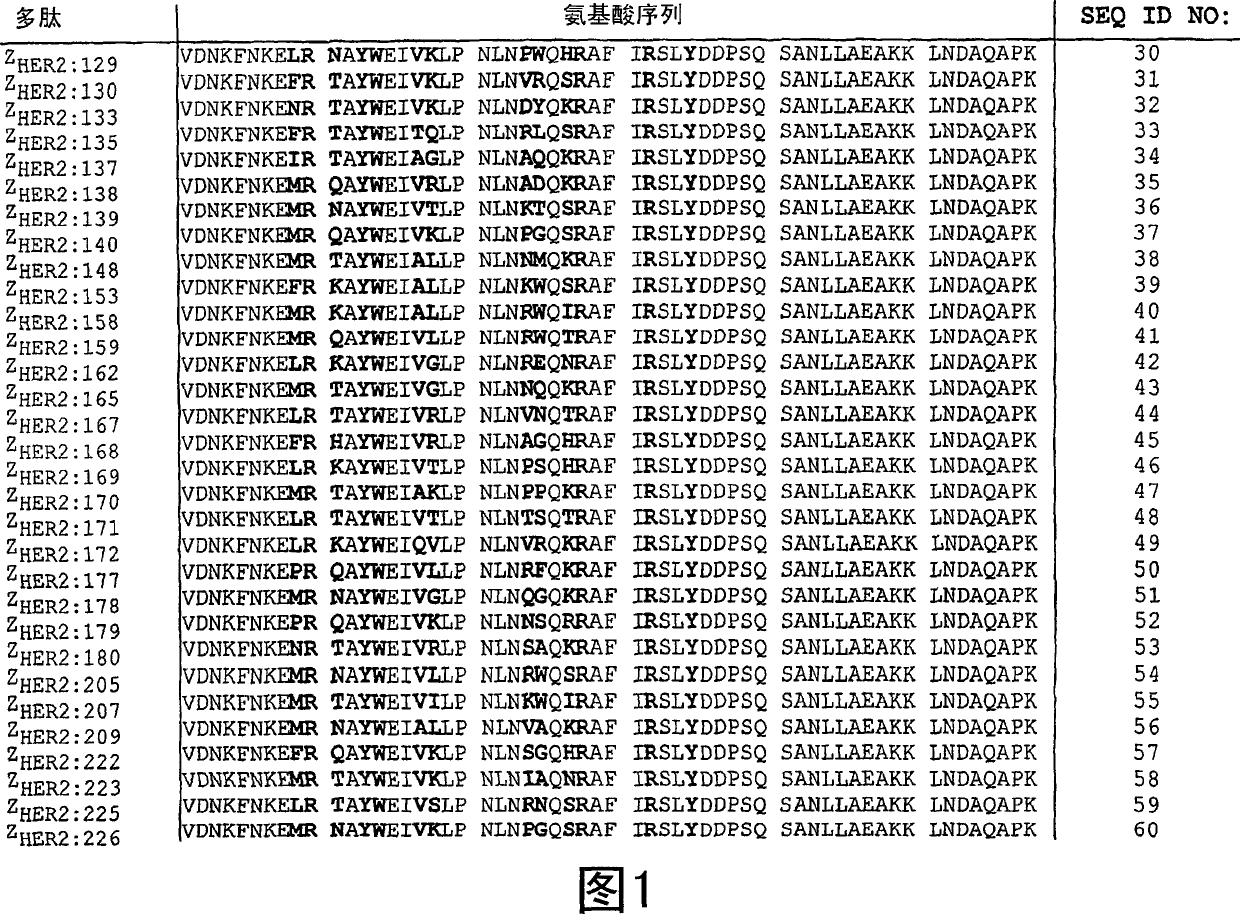
[0109] Selection and Research of HER2 Binding Polypeptides
[0110] In this experiment, multiple HER2-binding polypeptides of the invention were selected from a library containing many different SPA domain-related polypeptides and subsequently characterized. Library panning and clone selection
[0111] A combinatorial phage display library has been substantially prepared by Nord K et al (1995, supra). The library pool (pool) used in this study contains 8.7×10 8 A Z protein variant (Affibody molecule) having random amino acid residues at positions 9, 10, 11, 13, 14, 17, 18, 24, 25, 27, 28, 32, and 35. Using biotinylated human HER2 extracellular domain (HER2-ECD) as target (recombinant human HER2 extracellular domain, amino acids 238-2109, provided by Fox Chase Cancer Center, Philadelphia, USA) in four panning cycles Selection of Affibodies that bind antigens molecular. After these four rounds of selection rounds, a total of 91 clones were selected for ph...
Embodiment 2
[0124] Z HER2A Binding to HER2 expressing cells
[0125] cell culture
[0126] The human breast cancer cell line SKBR-3 was purchased from ATCC (ATCC #HTB-30), and it is known that each cell of this cell line expresses about 2×10 6 HER2 molecules. Cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM L-glutamine and PEST (100 IU / ml penicillin and 100 μg / ml streptomycin), all purchased from Biochrom KG (Berlin, Germany). Cells were cultured at 37°C in a humidified atmosphere containing 5% CO2 and seeded in 3 cm petri dishes three days before the start of the experiment.
[0127] Radiolabeled
[0128] Labeling precursor, N-succinimidyl p-(trimethyl-stannyl)benzoate (SPMB) according to Orlova et al, Nucl Med Biol 27 : 827-835 (2000) method is prepared, the SPMB of 5 μ g is added to containing 5MBq 125 I in 5% acetic acid solution. 40 μg of an aqueous solution of Chloramine-T (Sigma, St. Louis, MO) was added to initiate the re...
Embodiment 3
[0133] Expression and Characteristic Analysis of HER2 Binding Polypeptide Dimer
[0134] DNA vector construction and protein production
[0135] Select a new affibody ligand as above, His 6 -Z HER2A , which has an affinity for the HER2 receptor. by coding Z HER2A The gene fragment of the polypeptide is subcloned into His 6 -Z HER2A to construct the dimer Z in the expression vector of HER2 Variants. The DNA Sequencer ABI Prism The introduced Z was confirmed by DNA sequencing on the 3700 Analyzer (Applied Biosystems, Foster City, CA). HER2A fragment. Escherichia coli strain RR1ΔM15 (Rther, Nucleic Acids Res 10: 5765-5772 (1982)) was used as the bacterial host during the cloning process. The resulting vector encodes a dimeric Z under the control of the T7 promoter (Studier et al, Methods Enzymol 185:60-89 (1990)). HER2 Variant, (Z HER2A ) 2 , with N-terminal hexahistidyl (His 6 ) tag fusions that allow purification by immobilized metal ion affinity chro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com