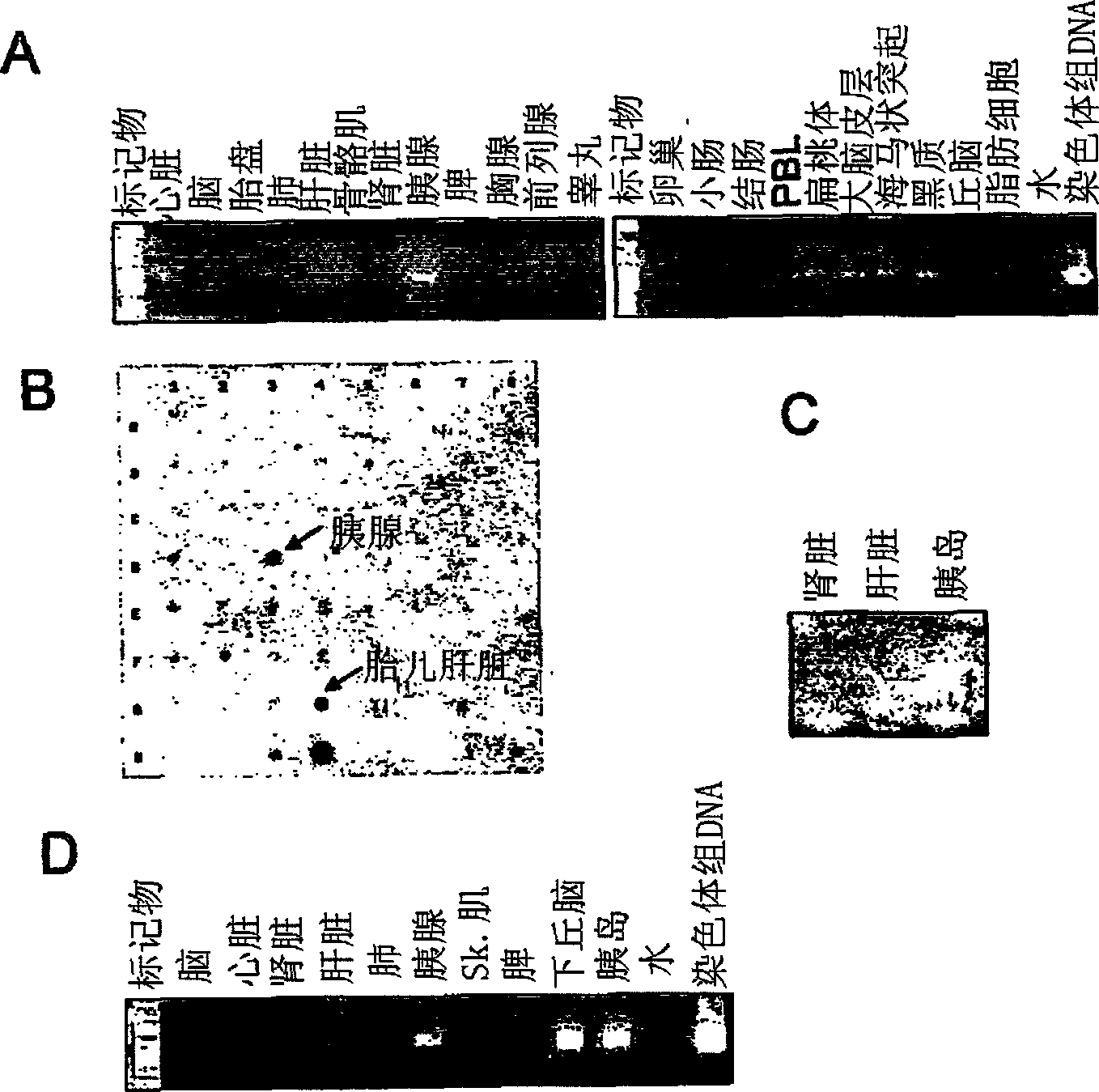
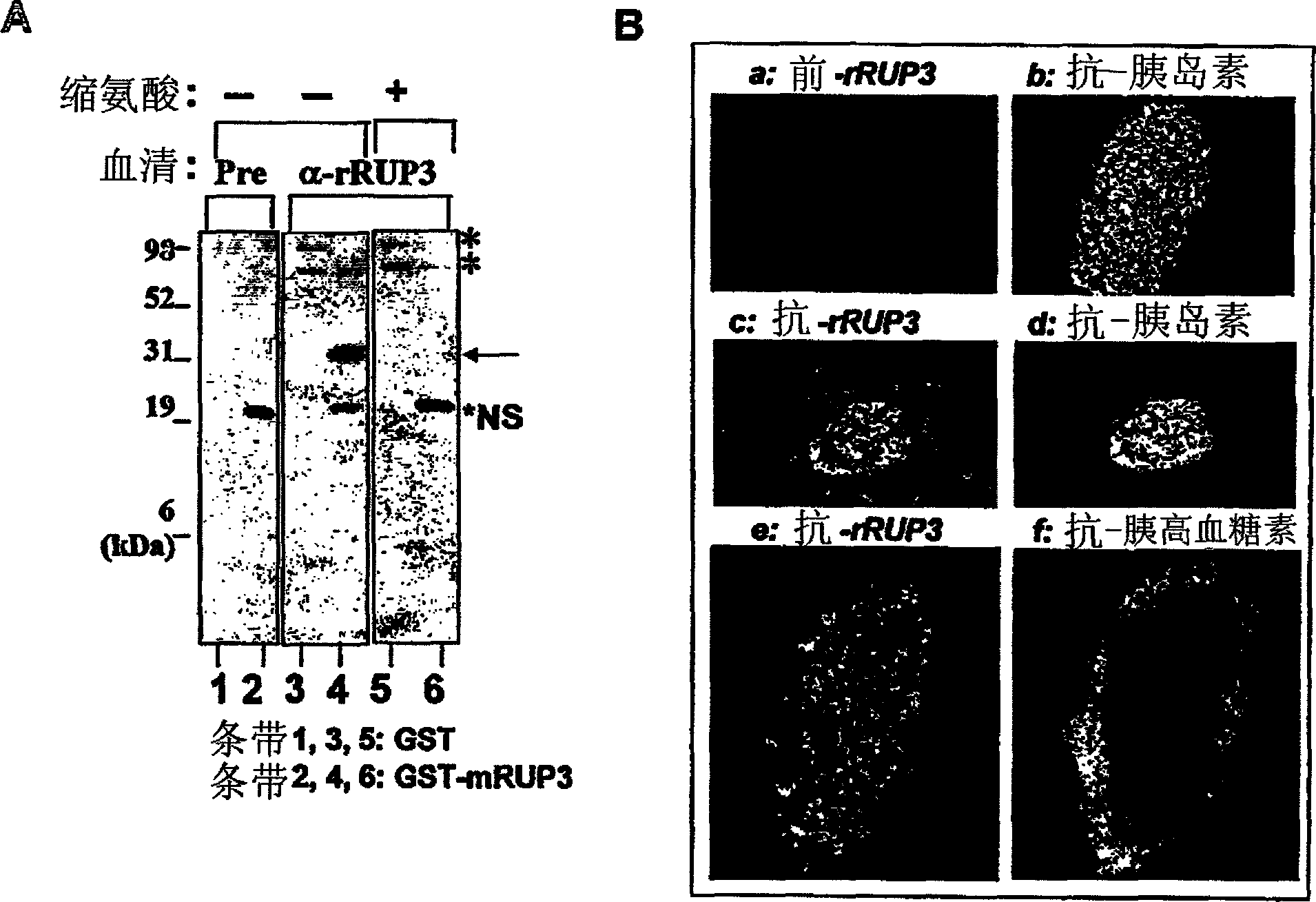
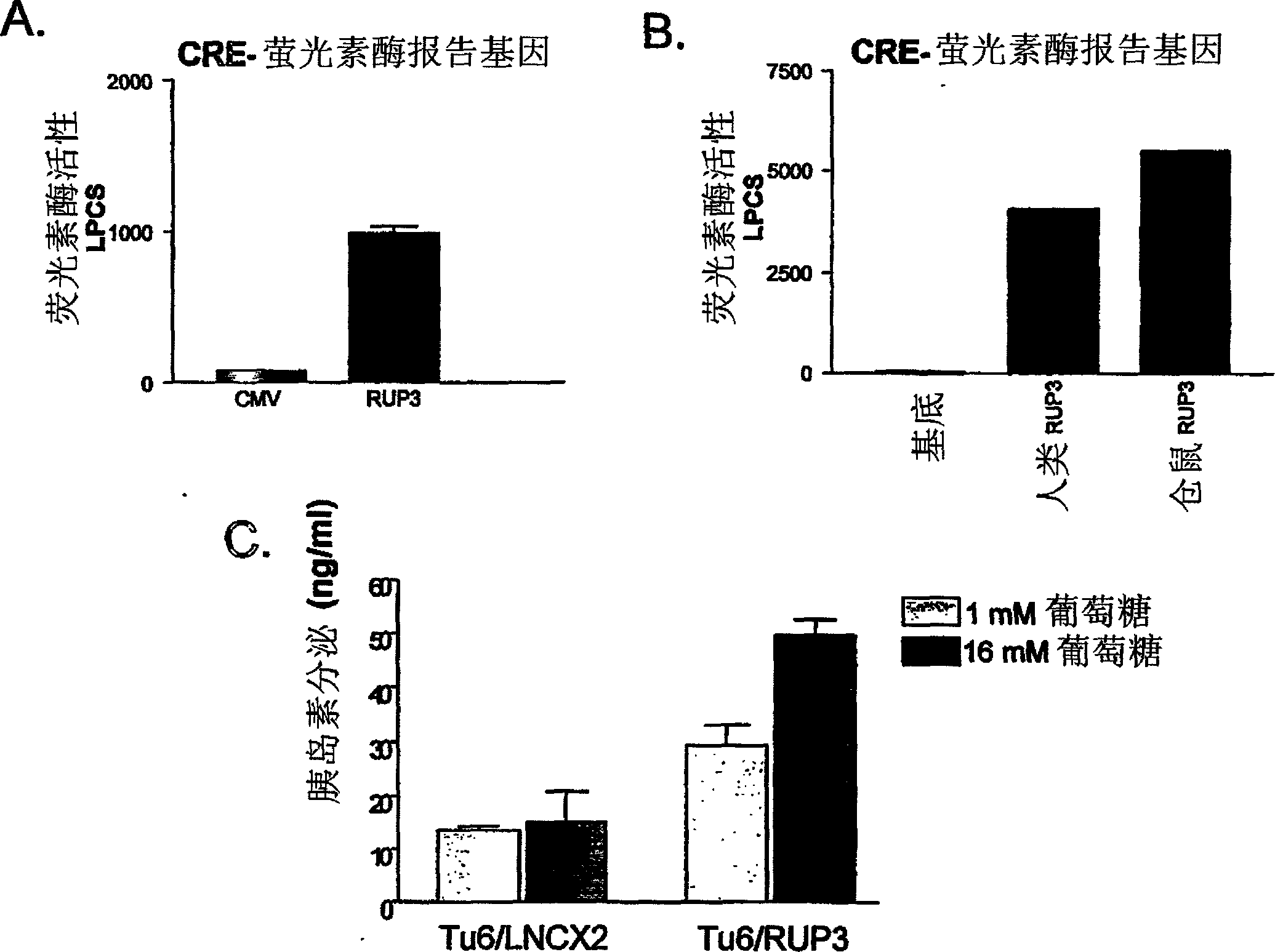
Fused-aryl and heteroaryl derivatives as modulators of metabolism and the prophylaxis and treatment of disorders related thereto
A compound and hydrate technology, applied in metabolic diseases, active ingredients of heterocyclic compounds, drug combinations, etc., can solve problems such as increased risk of death without taking weight ratio into account
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0958] The activity level 125 The synthetic methods for I binding to the target molecule include:
[0959] A. Sandmeyer and its analogous reactions-this procedure converts aromatic amines or heteroaromatic amines into (for example) the diazonium salt of tetrafluoroborate, and then uses Na 125 I convert it into 125 I-labeled compound. Zhu, D.-G. and their collaborators reported a representative procedure in J. Org. Chem. 2002, 67, 943-948.
[0960] B. Ortho position of phenol 125 Iodination-this program makes 125 I binds to phenol in the ortho position, as reported by Collier, T.L. and his collaborators in J.labelled Compd Radiopharm.1999, 42, S264-S266.
[0961] C. Aryl bromide and heteroaryl bromide and 125 I Interchange-This method is generally a two-step procedure. The first step is to use, for example, Pd to catalyze the reaction [ie Pd(Ph 3 P) 4 ] Or through aryllithium or heteroaryllithium in trihaloalkyl tin or hexaalkyl ditin [e.g. (CH 3 ) 3 SnSn(CH 3 ) 3 ] The presence of...
example
[0967] The examples are provided to further illustrate the present invention in detail without restricting the present invention to the details of these examples.
example 1
[0969] 96-well ring AMP membrane test of RUP3
[0970] material
[0971] 1) Purchasing Perkin Elmer's adenylate cyclase activation scintillation plate test kit-96-well (SMP004B) and the kit provided with the kit 125 I tracer (NEX130), placed in a box and stored in the refrigerator, and do not expose the scintillation plate to light.
[0972] 2) Creatine Phosphate-Sigma P-7936
[0973] 3) Creatine Phosphokinase-Sigma C-3755
[0974] 4) GTP-Sigma G-8877
[0975] 5)ATP-Sigma A-2383
[0976] 6) IBMX-Sigma I-7018
[0977] 7) Hepes-1M distilled water solution-Gibco#15630080
[0978] 8) MgCl 2 -SigmaM-1028-1M solution
[0979] 9) NaCl-Sigma-S6546-5M solution
[0980] 10) Bradford protein test kit-Biorad#5000001
[0981] 11) Proclin 300-Sigma#4-8126
[0982] Binding buffer -Filter through a 45-micron Nalgene filter and store in the refrigerator. All buffers and membranes should be kept cold (in an ice bucket) during testing.
[0983] 20mM Hepes, pH7.41 mM MgCl 2 100mM NaCl
[0984] 2×Reg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com