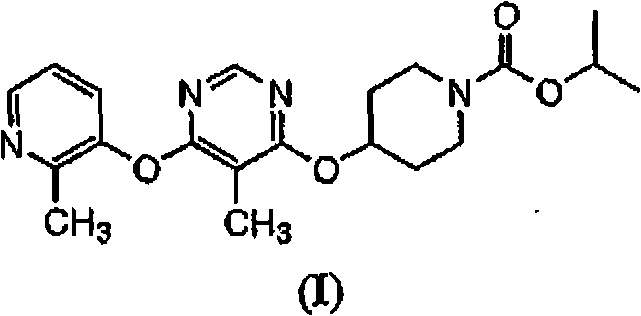
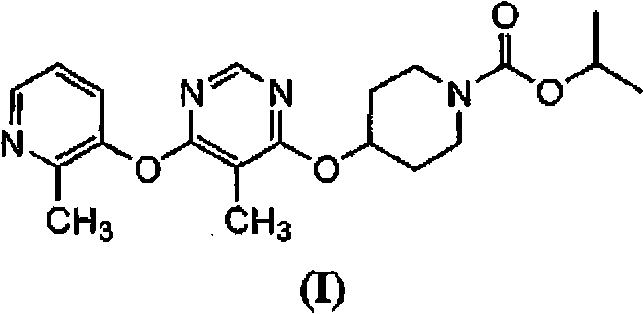
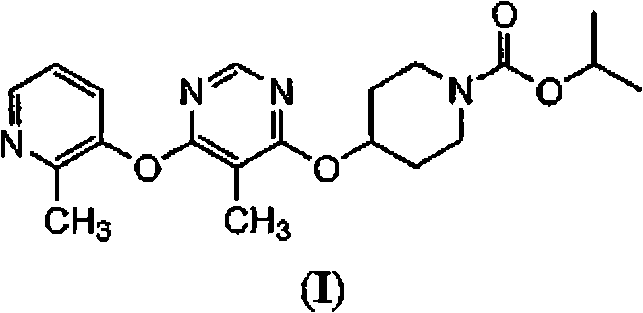
Modulators of metabolism and the treatment of disorders related thereto
A related disorder, carrier technology for metabolic modulators and the treatment of metabolic related disorders that addresses increased risk of death, no muscle-to-weight ratios to account for, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0140] Example 1: In vivo effects of RUP3 agonists on glucose homeostasis in rats
[0141] General Procedures - Oral Glucose Tolerance Test (oGTT)
[0142] Male Sprague Dawley rats (Harlan, San Diego, CA) weighing approximately 350-375 g were fasted for 16 hours and randomized (n=6) to receive 0.3, 3 or 30 mg / kg RUP3 agonist. Compounds were delivered orally (p.o., volume 2 mL / kg) via a feeding tube needle. At time 0, blood glucose concentrations were estimated using a glucometer (Accu-Chek Advantage, Roche Diagnostics), and vehicle (20% hydroxypropyl-β-cyclodextrin) or test compounds were administered to rats. Thirty minutes after administration of the test compound, the blood glucose concentration was assessed again, and dextrose was orally administered to the rats at a dose of 3 g / kg. Blood glucose values were measured at 30 min, 60 min and 120 min after this time. Table 1 shows the mean percent inhibition of glucose excursion for each test compound, averaged over 6 an...
example 2
[0146] Example 2: Receptor Binding Assay
[0147]In addition to the methods described herein, another method for evaluating test compounds is to determine binding affinity to the RUP3 receptor. This type of assay typically requires a radiolabeled ligand of the RUP3 receptor. Instead of using known RUP3 receptor ligands and their radiolabelling, compounds of formula (I) can be radioisotopically labeled and used in assays to evaluate the affinity of test compounds for the RUP3 receptor.
[0148] Radiolabeled compounds of formula (I) RUP3 can be used in screening assays to identify / evaluate compounds. In general terms, newly synthesized or identified compounds (ie, test compounds) can be assessed for their ability to reduce the binding of a "radiolabeled compound of formula (I)" to the RUP3 receptor. Thus, the ability to compete with a "radiolabeled compound of formula (I)" or a radiolabeled RUP3 ligand for binding to the RUP3 receptor is directly related to the binding affinit...
example 3
[0159] Example 3: CYP program
[0160] P450 inhibition screening assays were performed in 96-well microtiter plates using cDNA expressed human enzymes (CYP1A2, 2C9, 2C19, 2D6 and 3A4). Test compounds (prepared in acetonitrile) were serially diluted in phosphate buffer (pH 7.4) containing the electron generating system (glucose-6-phosphate, NADP+ and glucose-6-phosphate dehydrogenase). Enzyme reactions were initiated by the addition of each P450 enzyme premixed with a P450-specific fluorogenic substrate and terminated by adding acetonitrile (or NaOH for DBF analysis) to the reaction mixture after a certain period of incubation at 37°C. Fluorescence of metabolites was measured on a Biotek fluorescence reader. Results are expressed as percent inhibition relative to control (no test compound). Estimation of IC from concentration-response curve 50 value. P450 substrates that can be used in this assay include dibenzylfluorescein (DBF, for CYP2C9, 2C19, and 3A4), 3-cyano-7-ethoxy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com